Why Was Atomic Mass Scale Changed From Oxygen
Di: Everly
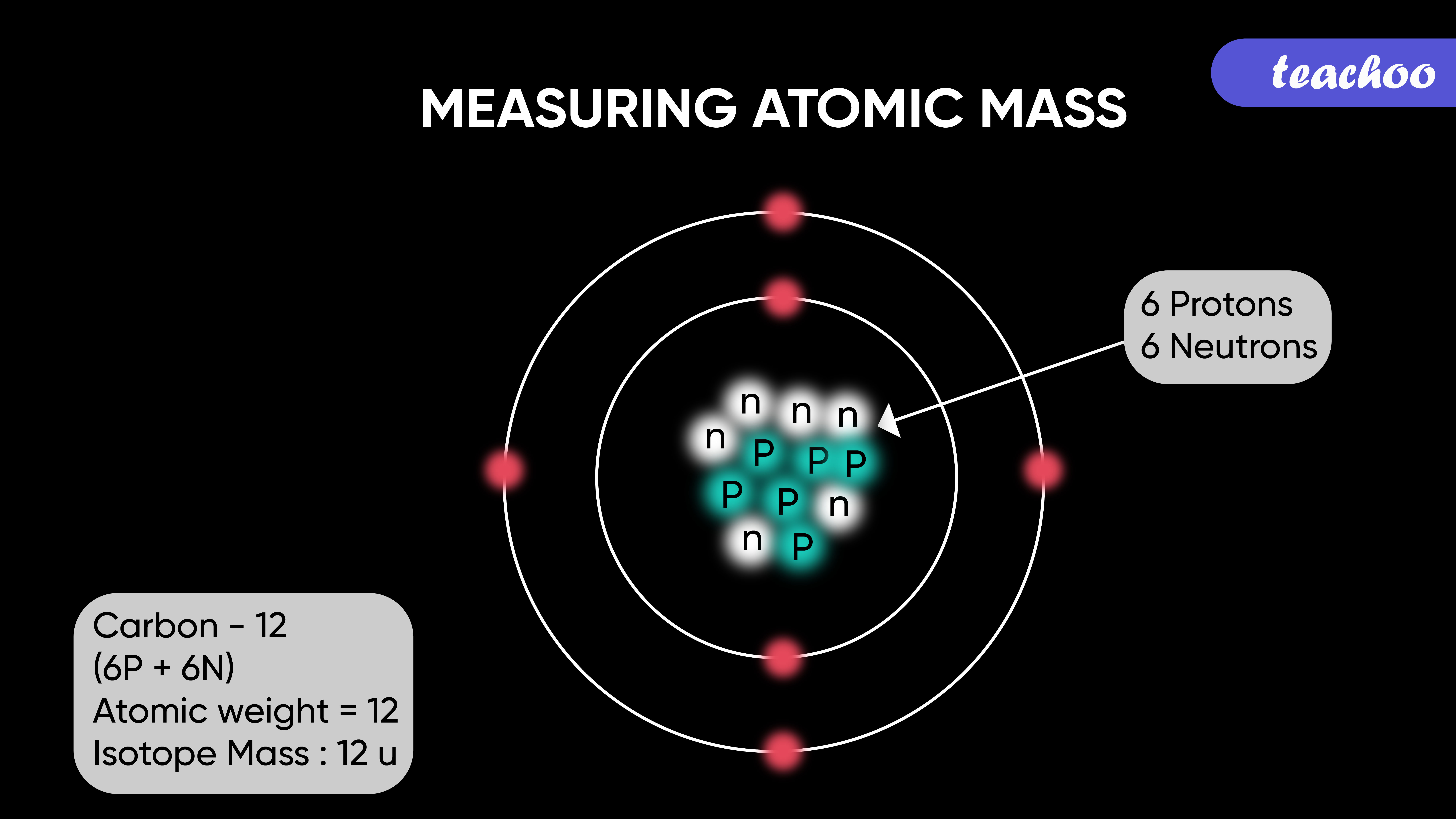
Why is Carbon-12 used as the measurement of relative masses?
Why atomic mass is unites What’s atomic mass? Atomic mass is the mass of an atom. However, when you look on the periodic table, you usually don’t see units attached to
Atomic mass (m a or m) is the mass of a single atom.The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus, with minor contributions from the
Why did we discard oxygen as a choice to measure atomic mass Get the answers you need, now! suggested to Josef Mattauch that the atomic weight scale be based on
From Avogadro’s hypothesis, however, each water molecule contains twice as many atoms of hydrogen as oxygen, so to achieve the experimental mass relationship, each
Research shows that 8 grams of oxygen combine with 1 gram of hydrogen. It follows, then, that each atom of oxygen has a mass eight times that of a hydrogen atom. This reasoning led to
- Why was oxygen-16 replaced by carbon-12 as the reference atom?
- Why was atomic mass scale changed from Oxygen
- Why oxygen was removed from being considered as the sta
- Evolution of the unified scale of atomic mass, 12C = 12u
For example, for our purposes here, we will say that a hydrogen atom has a mass of 1 on the atomic mass scale. Then an oxygen atom has a mass of 16 on this scale. Our conclusions
What’s atomic mass and why is it that it has no units?
In the years between when the mass scale change occurred and 1969, there were relatively few changes in the atomic weights table. In the 1969 report, a table of radioactive isotopes with half
When IUPAP and IUPAC agreed to change the atomic mass scale from oxygen to carbon in 1960/1961, the physicist’s definition of the atomic mass unit, amu, as equal to 1/16th
Improved measurements and more widespread usage made repeatability become a significant issue by the middle of 20th century. The primary cause is natural variations in the
Oxygen was not selected as the standard reference for atomic mass. Instead, the choice of the standard reference for atomic mass is based on the carbon-12 isotope. The
Since oxygen forms compounds with more elements than practically any other, oxygen was a natural standard for an atomic mass scale. Then, chemical analysis would allow one to
As a result, when calculated in daltons, the numeric value of the atomic mass is almost equal to the mass quantity. Since the chemical atomic weights of carbon 12 are almost equal to those of
Why did we discard oxygen as a choice to measure atomic mass
- Why is Carbon-12 the standard of Atomic Mass?
- Atomic Mass of ¹²C before 1961
- Comparison of oxygen and carbon-12 as standards for mass
- What’s atomic mass and why is it that it has no units?
- Videos von Why was atomic mass scale changed from oxygen
The Carbon-12 atom having an atomic mass of exactly 12u is just a consequence of the definition of the atomic mass unit. Nothing special about it. There are several actual
Physicists picked the pure isotope Oxygen-16, because they tended to make their measurements on the basis of mass spectrometry. Though the ratio of any two atom’s masses was the same
Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.
.jpg)
atomic mass scale, 16O was assigned an exact mass of 16.000000 amu, and all other atomic masses were defined rela- tive to this standard (that is, the amu is defined as 1⁄16 the mass of
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.
Why was carbon 12 chosen over oxygen for atomic mass if oxygen was so suitable? Open in App. Solution. Carbon is an element which can react with more elements than oxygen. Carbon can
Since oxygen forms compounds with more elements than practically any other, oxygen was a natural standard for an atomic mass scale. Then, chemical analysis would allow
One of ways of obtaining atomic mass is analyzing mass percentages of binary compounds where one of elements is considered as the atomic mass standard. Reactivity of
The chemists used a „atomic mass unit“ (amu) scale such that the natural mixture of oxygen isotopes had an atomic mass 16, while the physicists assigned the same number 16 to only the
It traces the sequential use of hydrogen, oxygen and 12 C as standards of chemical atomic weight, and of 16 O and 12 C as standards of physical atomic mass.
For example,Relative Atomic Mass of Oxygen= 16 u So,Gram atomic mass of Oxygen=16 g. Skip to main content. Stack Exchange Network. Stack Exchange network
The ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant is defined as relative atomic mass (A r) or atomic weight. The atomic mass
Therefore, they came up with the solution of carbon-12 as the standard in which the relative change in the atomic mass scale (42 ppm) is less than that of oxygen-16. The carbon-12 atom
Protons don’t have the same mass as neutrons and Carbon 12 isn’t exactly 3/4 the mass of Oxygen 16. This led to a need for a specific atomic mass unit. When there’s a unit there needs
Also, 12C = 12 implied acceptable relative changes in the atomic weight scale, i.e., 42 parts-per-million (ppm) compared to 275 ppm for the 16O = 16 scale (which would not acceptable to
This made it a better choice as a standard because of the ease of chemical analysis. Oxygen was set to have an atomic mass of 16, which Its this change in mass when the nucleus changes
Carbon-12 replaced oxygen-16 as atomic mass reference due to stability and isotopic consistency, improving precision in mass calculations. Oxygen-16 (^16O) was originally used as the
In 1961, the standard for the atomic mass unit (amu or u) was changed from using oxygen to using carbon-12 as the reference isotope. The reason for this change had to do with
$\begingroup$ On of reasons for carbon-12 was reportedly solving the metrological schisma where chemists were using the scale based on natural oxygen while
- Ausbildung Fachinformatiker 2024 Jobs In Hessen
- Kaufen Giana Sisters: Twisted Dreams
- Artillery Calculator For Hell Let Loose That Runs On Your Desktop
- Die Termin-Börse | Die Größten Terminbörsen
- Katzenpfötchen Pflanzen Und Pflegen
- 15 Best Electric Scooters In Australia
- Hardened Mana Enchantment – Hardened Mana Hypixel Skyblock
- Pankreasinsuffizienz Und Enzymsubstitution
- Restaurant Löwen Walenstadt Speisekarte
- Poe Farms History — Washington Wheat Foundation
- Whatsapp’ta Silinen Mesajları Okuma Yöntemi [Nasıl Yapılır?]
- Leifheit Wäscheständer Aluminium Blau
- Forests And Human Health: Forests For Human Health
- Barcelona: Vier Milliarden Euro Schulden
- Punkteregelung In Flensburg: Andere Bepunktung Seit 2014