What Is The Difference Between Molar Mass And Molecular Weight
Di: Everly
Mass moles are calculated by dividing the mass of a sample by its molecular weight, whereas molecular weights are calculated by multiplying the mass of each atom in a
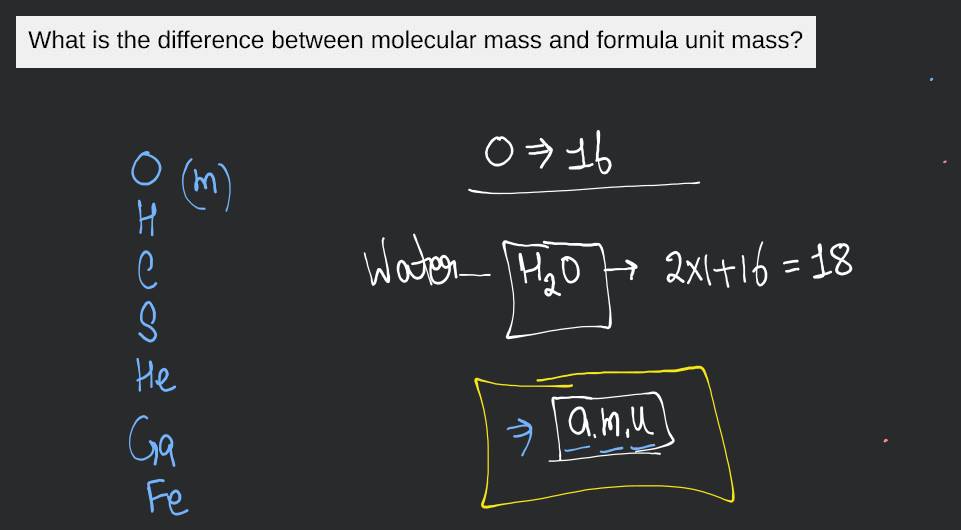
The molecular or formula weight expressed in grams per mole. The molar mass of water (H2O) is approximately 18 g/mol. 8. Formula Mass. A representation of the molecular
Molecular Weights of Polymers
Molecular weight can be defined as the average mass of a molecule of a compound compared to ¹/₁₂ the mass of carbon 12 (C – 12) and is calculated as the sum of the atomic weights of the
Molar mass is the mass of one mole of a substance, while molecular mass is the mass of one molecule. Molar mass is expressed in grams per mole, while molecular mass is
The molar mass of magnesium will thus be 24 g/mol. The relative molecular mass of carbon dioxide is 44. This means that one mole of carbon dioxide molecules has a mass of 44 g. The
- Gram Equivalent Weight vs. Gram Molecular Weight
- Understanding the Difference Between Molar Mass and Molecular Weight
- Difference between Molecular Mass and Formula Mass
- 6.2: Molecular Mass and Formula Mass
Atomic Weight & Molecular Weight Indicator Words. Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. Therefore,
Atoms of different elements having the same mass number but different atomic number are called isobars. Examples include tritium and helium-3. Atomic mass. The atomic mass of an isotope
Molecular weight, as a synonym of molar mass, is defined as the mass of a sample of that compound divided by the amount of substance in that sample, measured in moles (g/mol). The molecular
Equivalent Weight vs. Gram Equivalent
One mole (abbreviated mol) is equal to 6.022×10 23 molecular entities (Avogadro’s number), and each element has a different molar mass depending on the weight of 6.022×10 23 of its atoms
The main difference between molecular mass and molar mass is that molar mass is the weight of a mole of atoms or molecules, while molecular mass is the total of all the atoms in a molecule’s
Weight Average Molecular Weight (Mw) is another measure of the average molecular weight of a polymer sample, but it takes into account the relative abundance of different molecular weight
Molecular weight, mass of a molecule of a substance, based on 12 as the atomic weight of carbon-12. It is calculated in practice by summing the atomic weights of the atoms making up
Molar Weight and Molecular Weight. Mw should not be confused with molar weight (Mr), which is the mass of a single molecule and is thus independent of its environment. Molecular weight is
Weight and distance from Earth The weight of an object with a mass of 50 kg (110 pounds) will decrease as its distance from Earth’s center increases. (Earth’s surface is about
The main difference between molar mass and molecular weight is that molar mass gives the mass of a mole of a particular substance whereas molecular weight is the mass of a molecule of a particular substance.
Difference between relative atomic & molecular masses with molar mass
Sum the masses to find the molecular mass or formula mass. molecular mass = 342.3 amu: formula mass = 164.10 amu: Explanation. It is called a molecular mass since C 12 H 22 O 11 is
If you would like to know about What is the relationship between mass moles and molecular weights,which explains the difference between moles and molecular weights.
In a nutshell, a molecular mass refers to the total mass of a substance. It sums the average masses of individual atoms in a molecule of that particular substance.
The molar mass and molecular weight are equivalent. The primary distinction is that molecular weight solely refers to the relative weight of molecules relative to elements. The

Molar mass is the mass of a mole of a substance (measured in g/mol) while molecular mass is the mass of one molecule (measured in atomic mass units). For example,
While atomic mass and molar mass are numerically equivalent, keep in mind that they are vastly different in terms of scale, as represented by the vast difference in the magnitudes of their
Solutions to Example 6.2.2, Multiplying the molar mass of each atom by the number of atoms of that type in bilirubin’s formula and adding the results, we get; 33 C molar mass: 33 × 12.01 g
Molar Mass is the mass of one mole of a substance, measured in grams per mole (g/mol). Molecular Weight is the sum of the atomic weights of atoms in a molecule, measured in atomic mass units (amu).
The molar mass of any substance is its atomic mass, molecular mass, or formula mass in grams per mole. The periodic table lists the atomic mass of carbon as 12.011 amu; the average molar
For example, the relative molecular mass of water (H2O) is approximately 18 because it is made up of two hydrogen atoms (each with a relative atomic mass of 1) and one oxygen atom (with a
- Ausschläger Weg Hamburg 2024 | Ausschläger Weg Hamburg
- Bühnen-Geständnis: Ex-Pbb Dominik Bruntner Ist Verliebt!
- Makino Nachos, Nuts
- Joop! Go, Eau De Toilette, 50 Ml
- Hpg 145 Action Pack Download – Hpg H145 Action Pack Download
- Plattdeutsch Für Senioren – Plattdeutsche Lieder Zum Mitsingen
- Rainbow Six Siege Operators By Picture Quiz
- In Den Hausäckern 75385 Bad Teinach-Zavelstein
- E Din Iec 60335-2-69 Vde 0700-69:2024-08
- „Sissi“-Trilogie: Diese Film-Momente Sind So Nie Passiert
- Cinque Women Bluse Cithalo Ci-5652-2227-75-231-32 Grün
- Honkai: Star Rail – Honkai Star Rail Home Page
- Técnicas De Ventas Efectivas Para 2024 [Basadas En Datos]