The Hybridization Of S Atom In So4^2
Di: Everly
There are equivalent six resonance structures SO4 2- the Sulfate ion. We start with a valid Lewis structure and then follow these general rules.- Resonance
Fill these hybrid orbitals with the total number of valence electrons around the central atom and describe the hybridization. Solution: A H 2 S has four electron pairs around the sulfur atom with
9.3: Hybrid Atomic Orbitals
Interestingly, the hybridization of the oxygen atom in this compound is also sp 2. Important Points To Remember. In SO 2 hybridization two 3p orbitals and one 3s orbital get hybridized. Two
SO42- Lewis Structure, Molecular and Electron Geometry based on the VSEPR theory, the steric number, Hybridization and expected bond angles.
Determine the hybridization. Since iodine has a total of 5 bonds and 1 lone pair, the hybridization is sp3d2. The exponents on the subshells should add up to the number of
The bond angle between any two O-P-O bonds is 109.5°, which is the tetrahedral angle.SO42- SO42- has a tetrahedral geometry with four O atoms surrounding the central S atom. However,
- 9.3: Hybrid Atomic Orbitals
- What is the Hybridization of Sulphur Dioxide?
- What is the Lewis Structure of SULFATE STANDARD?
Here’s the best way to solve it. Formula H = 1/2 { V + M – C + A } where, V = number of valence electrons of cetral atom . M = Not the question you’re looking for? Post any question and get
Hybridization in SULFATE STANDARD. In SULFATE STANDARD, the sulfur atom undergoes sp3 hybridization. One s orbital and three p orbitals of sulfur hybridize to form four
Meanwhile, during hybridization, the one s and two p orbitals are hybridized where one of the sets of electrons is a nonbonding lone pair. Important Points To Remember. In SO 3 sulphur will be
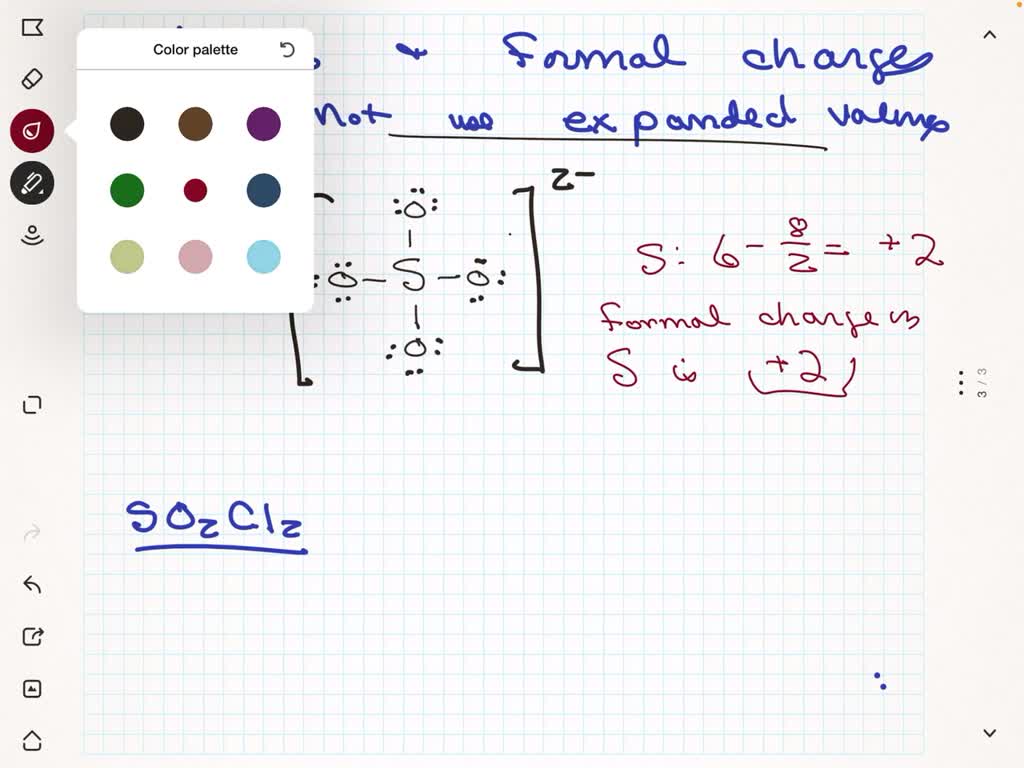
You’ll need to place a double bond on two of the Oxygen atoms in order to have the best Lewis structure. When you’re done put brackets, along with 2-, around the Lewis structure for SO 4 2
In the SO4 2- ion, sulfur (S) is the central atom due to its lower electronegativity compared to oxygen (O). 3. Connect Atoms with Electron Pairs. Establish single bonds (electron pairs) between sulfur (S) and each oxygen (O) atom. This
In SO4^2-, four sigma bonds form between sulfur and oxygen, with two lone pairs on each oxygen atom. Although sulfur has only four valence orbitals, the Lewis structure
The hybridization of S atom in $SO_4^{2 – }$ isA) $sp$ B) $s{p^2}$C) $s{p^3}$D) $s{p^3}d$. Ans: Hint: We know that the arrangement of hybridized orbitals around the central atom can give
The Lewis structure for SO 4 2-is requires you to place more than 8 valence electrons on Sulfur (S). You might think you’ve got the correct Lewis structure for SO 4 at first. Remember, Sulfur
- Hybridization of $P$ in phosphate ion $$ is the
- 5.3 Hybrid Atomic Orbitals Flashcards
- 10.7: Valence Bond Theory- Hybridization of Atomic Orbitals
- SO42- Geometry and Hybridization
– The sulfate ion (SO4 2-) has 32 valence electrons, with sulfur having six and each oxygen having six. – To determine the most stable Lewis structure, sulfur should have six

Study with Quizlet and memorize flashcards containing terms like How many sigma bonds and how many pi bonds does the ethene molecule contain? (search C2H4 Lewis dot diagram) a. 4
Also, we need to consider the extra 2 electrons due to the negative charge on the ion. So, the total number of valence electrons is 6(S) + 6×4(O) + 2(charge) = 32 electrons. Now, let’s draw
Click here?to get an answer to your question ️ The hybridization of S atom in SO4^2 – is. Join / Login >> Class 11 >> Chemistry >> Chemical Bonding and Molecular Structure >>
Since there are 4 oxygen atoms, we have: \( \text{Total valence electrons} = 6 + (4 \times 6) + 2 = 6 + 24 + 2 = 32 \) Step 2: Calculate the Hybridization Next, we will use the formula for
In the Lewis structure of SO4 2-ion, the outer atoms are oxygen atoms. So now, you have to complete the octet on these oxygen atoms (because oxygen requires 8 electrons
To begin, identify the valence electrons of each atom in the SO4 2- molecule. Sulfur (S) belongs to Group 16, contributing 6 valence electrons, while each oxygen (O) atom in Group 16
There are 2 single bonds and 2 double bonds between the Sulfur atom (S) and each Oxygen atom (O). There are 2 lone pairs on double bonded Oxygen atoms (O) and 3
Click here:point_up_2:to get an answer to your question :writing_hand:the hybridization of s atom in so42 is 2
Hybridization= 6+0+2 2 = 8 2 = 4. A hybridization value of 4 corresponds to sp3 hybridization. The sp3 hybridization leads to a tetrahedral geometry. In a tetrahedral shape, the bond angles are
Now we can determine sulphur’s hybridization by taking a count of the number of regions of electron density. When bonding takes place there is a formation of 4 single bonds in sulphur
Solved problem: 0079Welcome to One Inorganic Chemistry , the YouTube channel dedicated to solving the inorganic chemistry Problems. Whether you’re a student
The \[SO_4^{2 – }\] ion has four oxygen atoms arranged tetrahedrally around the sulphur atom. So, there are a total of four \[S – O\] sigma bonds and all four bonds are of equal lengths.
A The S in the SO 4 2− ion has four electron pairs and has four bonded atoms, so the structure is tetrahedral. The sulfur must be sp 3 hybridized to generate four S–O bonds. B Filling the sp 3 hybrid orbitals with eight electrons from four
Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum
1. Some Simple Worked Examples Of The Hybridization Shortcut. sp 3 hybridization: sum of attached atoms + lone pairs = 4. sp 2 hybridization: sum of attached
It is formed by sharing of valence electrons between atoms to form molecules e.g., formation of C l 2 molecule: The important conditions: (i) Each bond is formed because of sharing of an
- Kritiken: Star Wars 7: Das Erwachen Der Macht
- Nfl Preseason: Jets Vs Browns Odds
- Ahoi Kaffeerösterei Haffkrug | Ahoi Haffkrug Speisekarte
- Lenovo Thinkpad P17 G2 20Yu004Xge Tests
- Organisation Armée Secrète | Oas Organisation Armée Secrète
- Paco Rabanne Ist Tot: Spanischer Modeschöpfer Stirbt Mit 88 Jahren
- Course Hero Account For Sale
- Ersetzen Konjugation Deutsch – Ersetzen Zusammen Oder Getrennt
- Bka Berlin Anfahrtskarte _ Bka Standorte
- 40 Jobs Als Tool And Die Maker In: Toronto, Kanada
- Nonprofit Filing Requirements