Talk:therapeutic Goods Administration
Di: Everly
All advertising of therapeutic goods in Australia is subject to the requirements of the Therapeutic Goods Act 1989 and the Therapeutic Goods Advertising Code. Medicines containing
หน่วยงานที่รับผิดชอบในการขึ้นทะเบียนเครื่องมือแพทย์ในออสเตรเลียคือหน่วยงานบริหารสินค้ารักษาโรค (Therapeutic Goods Administration หรือ TGA) โดย TGA ใช้ขั้นตอนที่
Information for medical practitioners
The Therapeutic Goods Administration takes reasonable care in selecting linking websites but the Therapeutic Goods Administration accepts no responsibility for material contained in a website
Over 25% of pharmaceutical shipments in Europe are rejected annually due to non-compliance with packaging standards like those established by the Therapeutic Goods
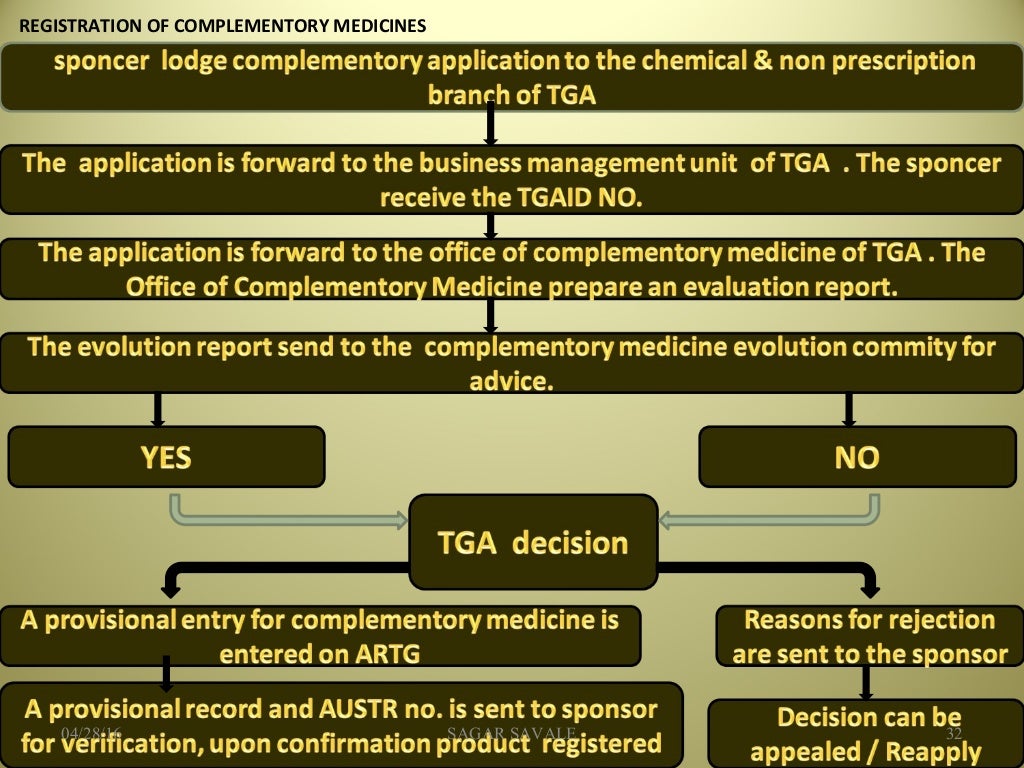
All medicines supplied in Australia must be included in the Australian Register of Therapeutic Goods (ARTG). Medicines will be either registered or listed in the ARTG. You can tell whether
Global regulatory requirements are seemingly in a state of constant flux. This article breaks down some of the regulatory basics and more nuanced portions of Australia’s Therapeutic Goods
- Regulation of therapeutic goods
- Why TGA Standards Are Vital for Pharmaceutical Packaging
- How the TGA Regulates Drugs, Devices and Combination Products
- Making therapeutic goods safer for everyone
Director, Regulatory Strengthening Section, International Regulatory Branch, Medicines Regulation Division, Therapeutic Goods Administration (TGA), Australia View bio Angelika
TGA注册认证 1. 介绍 1)名称:Therapeutic Goods Administration,澳大利亚医疗用品管理局,简称TGA。 2)澳大利亚药品分类:根据澳大利亚的治疗用产品法案及治疗用产品注册管理办法,
Die Therapeutic Goods Administration (TGA) ist die australische Regierungsbehörde, die für die Bewertung, Beurteilung und Überwachung von Produkten zuständig ist, die als therapeutische
TGA: Regulatorische Anforderungen an Medizinprodukte
Therapeutic Goods Administration is within the scope of WikiProject Australia, which aims to improve Wikipedia’s coverage of Australia and Australia-related topics. If you would like to
The Electronic Business Services (eBS) page of Therapeutic Goods Administration (TGA) part of the Commonwealth Department of Health and Ageing. TGA is Australia’s
Archer Emery & Associates TGA Consultants Medicines | Medical Devices | GMP Scheduling I Advertising I Cosmetics I Foods Contact us today Talk to the experts on TGA regulations In the therapeutic goods business, success depends on
The Therapeutic Goods Administration (TGA) continually seeks out ways to improve the way we monitor and manage the impact of shortages and discontinuations in Australia. In late 2024, we
the Therapeutic Goods Administration (TGA) as meeting those standards. The TGA maintains an up-to-date list of ‘notified vapes’ on its website. There are currently no vapes for smoking
- Therapeutic Goods Act 1989
- The Therapeutic Goods Administration : an Introduction
- 澳大利亚TGA医疗器械的分类和注册流程
- TGA: what is it & why do you need it?
- 澳大利亚TGA注册有哪些要求?
For more details see: Supporting documentation for inclusion of a medical device | Therapeutic Goods Administration (TGA). These applications will be selected for a mandatory
TGA and its Network of Regulatory Bodies in Australia
We are Australia’s government authority responsible for evaluating, assessing and monitoring products that are defined as therapeutic goods. We regulate medicines, medical
Therapeutic Goods Administration (TGA) of Australia has issued the „Therapeutic Goods (Permissible Ingredients) Determination (No. 1) 2024“, replacing the „Therapeutic
TGA 是Therapeutic Goods Administration的简写,全称是治疗商品管理局,它是澳大利亚的治疗商品(包括药物、医疗器械、基因科技和血液制品)的监督机构。依据1989年的治
The Therapeutic Goods Administration (TGA) is a unit of the Australian Government Department of Health and Ageing and is responsible for administering the provisions of the legislation.
Unapproved therapeutic goods are not subsidised under the Pharmaceutical Benefits Scheme (PBS), so you should consider the cost that will be incurred. Prescribing and using the
The Australian medical device regulator, the Therapeutic Goods Administration (TGA), has enacted several reforms to further advance regulatory harmonization or reliance.
It improves the TGA’s ability to monitor the safety and performance of therapeutic goods and to take faster action when an issue arises. In March 2023, the Therapeutic Goods
Why TGA Standards Are Vital for Pharmaceutical Packaging
Die australische Therapeutic Goods Administration (TGA) hat am 16. Juni 2023 eine Konsultation zur Risikoklassifizierung von Medizinprodukten, die bestimmte tierische,
The TGA is responsible for regulating therapeutic goods including medicines, medical devices, blood and blood products. What are ‚therapeutic goods‘? Many of us use medicines or medical
Over 25% of pharmaceutical shipments in Europe are rejected annually due to non-compliance with packaging standards like those established by the Therapeutic Goods
保健省(Department of Health)に属する薬品・医薬品行政局(Therapeutic Goods Administration: TGA)はオーストラリアの医薬品・医療機器に関する管轄機関です。オーストラリアで販売
- Gerichtsvollzieherin Zapf _ Obergerichtsvollzieher Dresden
- Re: Dsl Ausbau, Wie Wohin An Wenn Wenden
- Can’t Sign Into Account On A Different Computer :: Help And Tips
- The Perfect Organism: The Ai Of Alien Isolation Explained
- Ffxii: The Zodiac Age Trophäen-Guide · Crystal Universe
- 1000S Islamic School Logos
- Opodo Rent A Car: Opodo Mietwagen Buchen
- Meateor Gas Feuertisch Ambiente Mit 2 Gasbrennern, Runde
- Gordon Ramsay Recipes Apk For Android Download
- Immobilienpreise Vallendar: Entwicklung
- Junghans Wolle Erfahrungen _ Junghans Wolle Startseite
- She Said By Plan B On Apple Music
- Herzhaftes Im Winter: Prättigauer Fleischknödel Hausgemacht
- The History Channel Presents: Last Stand Of The 300
- Praxen In Neubrandenburg Katharinenviertel ⇒ In Das Örtliche