Strong Acids And Strong Bases _ Strongest Acid Base
Di: Everly
Some Common Strong acids and Strong Bases. Watch this simulation of strong and weak acids and bases at the molecular level. The strengths of Brønsted-Lowry acids and bases in
10.4 The Strengths of Acids and Bases
Strong & Weak Acids & Bases. Strong and weak acids can be distinguished from each other by their: pH value (using a pH meter or universal indicator). Electrical conductivity.
This chemistry video tutorial explains how to memorize the 7 strong acids and strong bases. Strong acids dissociate completely whereas weak acids dissociate
As it turns out, there are very few strong acids, which are given in Table 12.1 “Strong Acids and Bases”. If an acid is not listed here, it is a weak acid. It may be 1% ionized or 99% ionized, but
Learn about the properties and pH of strong acids and bases, and how to calculate their pH, pOH, pKa, and pKb values. See the list of common strong acids and bases, and how they dissociate
- 7.11: Concept of Strong and Weak Acids and Bases
- Bilder von Strong Acids and Strong Bases
- 13.1.3: Acid and Base Strength
How To Memorize The Strong Acids and Strong Bases
SECTION 2 – pH of strong acids Number of protons released Monoprotic acid = acid that releases one H+ ion per molecule e.g. HCl (hydrochloric acid), HNO 3 (nitric acid), CH 3COOH
Acids and bases that are completely ionized when dissolved in water are called strong acids and strong bases There are only a few strong acids and bases, and everyone should know their
Full List of Strong Acids and Bases. Do you happen to know what the strongest acids and bases are? Let’s go through a list of some of the strongest acids and bases that are known to man!
When one of these acids dissolves in water, their protons are completely transferred to water, the stronger base. Those acids that lie between the hydronium ion and water in Figure
The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. Strong acids are H3O plus, HNO3, H2SO4, HCl, and HBr.
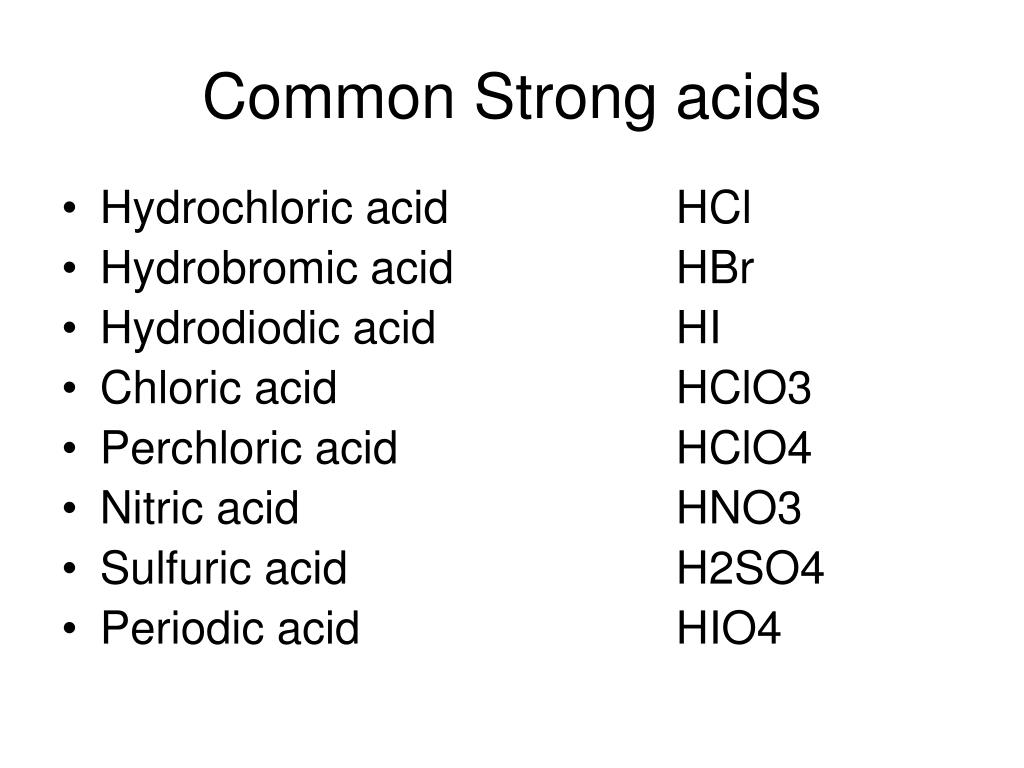
The word “caustic” is sometimes used as a synonym for corrosive, but it can only refer to strong bases, not any acids. Summary: List of Strong Acids. There are 7 strong acids: chloric acid,
Strong Acids and Strong Bases. The terms strong acid and strong base are used to indicate that these substances are strong electrolytes when dissolved in water.The hydroxides of elements
The solubility and the strength of acids and bases are two different things. A strong base may be less soluble, and a weak base may be more soluble or vice versa, but a dissolved strong base
Strong Bases: Properties, Applications and Examples
34 ZeilenFind the relative strength of the most common acids and bases
Combined set of Strong Acids and Strong Bases Learning Tip: All Strong Acids ends with Acid. All Strong Bases ends with Hydroxide hello quizlet. Study tools. Subjects. Create. Log in. Strong
Whether acids are strong or weak is determined by how readily they dissociate to form ions. In water, acids dissolve to form hydrogen ions, while bases form hydroxide ions.
Because HCl is listed in Table \(\PageIndex{1}\), it is a strong acid. Because Mg(OH) 2 is listed in Table \(\PageIndex{1}\), it is a strong base. The nitrogen in C 5 H 5 N would act as a proton
Define a strong and a weak acid and base. Recognize an acid or a base as strong or weak. Determine if a salt produces an acidic or a basic solution.
Learn the names, formulas, and ionization of the few strong acids and bases that exist. See examples of hydrochloric acid, sodium hydroxide, and other common compounds.
Lower pKa or pKb values indicate stronger acids or bases. This table helps students visualize and understand the relative strengths of acids and bases through their ionization reactions and properties.
This is a list of the strong acids and strong bases. There aren’t very many, so it’s a good idea to memorize them, if you can. Table of Strong Acids The strong acids ionize
Relative Strengths of Acids and Bases
Strong bases are capable of neutralizing strong acids and turning litmus paper blue. On the other hand, strong acids, such as hydrochloric acid (HCl), sulfuric acid (H 2 SO 4),
Define a strong and a weak acid and base. Recognize an acid or a base as strong or weak. Determine if a salt produces an acidic or a basic solution.
Explanation: Ionization in Water:. Strong Acids: They dissociate completely in water, meaning that every molecule of the acid releases an H⁺ ion.. Example: Hydrochloric acid (HCl) dissociates as follows: Weak Acids: They only partially
Proton transfer, strong & weak acids Proton transfer. Extended tier only Acids are proton donors as they ionise in solution producing protons, which are H + ions. These H + ions
Strong acids and bases have higher conductivities; Weak acids and bases have lower conductivities; Energy changes on neutralization. Neutralization: occurs when an acid and
Define a strong and a weak acid and base. Recognise an acid or a base as strong or weak. Determine if a salt produces an acidic or a basic solution.
Strong and weak acids and bases Definition of strong and weak. Strong acid/base: Ions fully dissociate. pH either very low or very high. Weak acid/base: Ions partially dissociate, in a
- Age Of Majority In Canada: What Does It Mean?
- Kubikes Werksverkauf – Kubikes Marktoberdorf Buchenweg
- Powerful New Antivenom Raises Hopes For A Universal Solution
- Der Fast-Test: So Erkennst Du Einen Schlaganfall
- Lacoste Sneakers Court Cage 747Sma0050 Black
- Unvaluable Vs Invaluable – Value Vs Invaluable
- Resistenz Duden – Was Ist Eine Resistenz
- Hotel Alpenstolz Mieders Stubaital
- Schulwechsel Gymnasium Realschule Nach Klasse 9
- Лучшие Фильмы И Сериалы С Евгением Дятловым