Solved Question 8 How Many Electrons Are Called ‘S, Electron
Di: Everly
The magnetic quantum number (m l) refers to the electron orbitals, and the electronic spin number (m s) refers to the electron spin state of the electrons. Each orbital can house 2 electrons, each
7.3: Electron Configurations of Atoms
For anions, the charge tells how many extra electrons there are compared to the number of protons. Recall, this pattern for the formation of anions and cations: Metals tend to lose
Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. The electrons in the innermost shell are the
These fundamental particles live for a really, really long time – 5 quintillion times as long as the Universe has existed, to be exact.
- Protons Neutrons & Electrons of All Elements
- Solved Student Exploration: Ionic Bonds Vocabulary: chemical
- 6.4 Electronic Structure of Atoms
- QUESTION 8How many electrons are called ‚ s ‚
Our expert help has broken down your problem into an easy-to-learn solution you can count on. Here’s the best way to solve it.
The atomic number of oxygen is 8, implying that an oxygen atom holds 8 electrons. Its electrons are filled in the following order: K shell – 2 electrons. L shell – 6 electrons. Therefore, the electron configuration of oxygen is 1s 2 2s 2
PROBLEM \(\PageIndex{2}\) Describe the properties of an electron associated with each of the following four quantum numbers: n, l, m l, and m s. Answer. n determines the general range for the value of energy and the probable
electronic structures of atoms
What is an electron shell with a model and diagram. Learn how many electrons are found in each shell with the rule, order, and electron configuration
Electrons are one of three main types of particles that make up atoms. Unlike protons and neutrons, which consist of smaller, simpler particles, electrons are fundamental particles that
Some are spherical, but other shapes also occur. While it’s theoretically possible to find an electron in the atomic nucleus, the highest probability of finding one is within its shell.
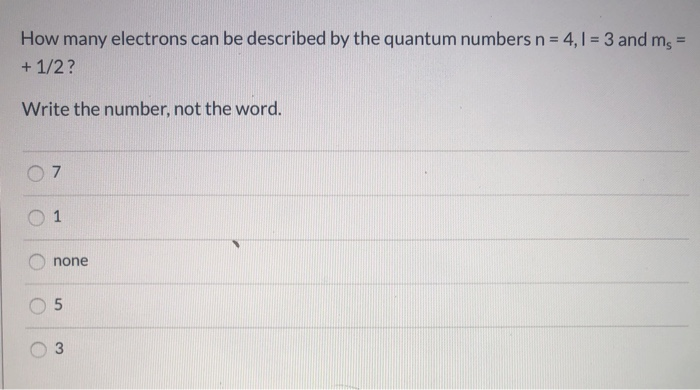
There are two s-electrons in the first energy level, two in the second energy level, and one in the third energy level. Thus there are five s-electrons in total. Find step-by-step Physics solutions
Study with Quizlet and memorize flashcards containing terms like What is the abbreviated electron configuration of yttrium (Y), The electrons in the outermost shell of an atom are
Question: QUESTION 6 Give the complete electron configurations in spdf notion of iron (Fe, atomic number 26 1s 2s22p63s23p63d64s2 1s22822p63s23p63d74s ls22s22p63s23p63d54s2
Unit 1 Review Questions Flashcards
Solved Example of Electronic Configuration. Example 1: Find the electronic configuration of oxygen (atomic number 8). Solution: The atomic number of oxygen is 8, which means it has 8 electrons. The electronic configuration of
Study with Quizlet and memorize flashcards containing terms like 1. How many electrons must be present before an atom has a second shell? a. 2 b.3 c.8 d.11 2. Select the statement that best
- What are Atomic Orbitals?
- What are Electron Configurations?
- How electrons solved the secret of eternal life
- 8.2 The Nature of Covalent Bonding Flashcards
- Unit 1 Review Questions Flashcards
The general formula for determining how many electrons can be housed in each shell is 2n 2 and is shown in Table 2.4. While this table predicts that that outer shells, which are the largest
Why is the electron in a Bohr hydrogen atom bound less tightly when it’s electron is in energy level 3 than when it is in energy level 1? Answer. An n of 3 indicated that the 1 electron in the

Electrons further away from the nucleus will have higher energy. An atom’s electron shell can accommodate 2n 2 electrons, where n is the energy level. For example, the first shell can
How many electrons are there in one coulomb charge?
Study with Quizlet and memorize flashcards containing terms like What are the three principle parts of an atom, and what charge does each carry?, How many times smaller is an electron
VIDEO ANSWER: Suppose a scientist repeats the Millikan oil-drop experiment but reports the charges on the drops using an unusual (and imaginary) unit called the
Each electron shell has one or more sub-levels, called subshells. The subshells are represented by the letters like s, p, d, f, g, h, and i. For example, the first (K) shell has one
Electron orbitals are mathematical functions that describe the probability of finding an electron around the nucleus of an atom. Each orbital can hold two electrons. They are also known as
How many core electrons are there? How many valence electrons? How do you draw the shell diagram of sodium atom? Answer. Sodium (Na) has atomic number 11, hence, 11 electrons. The electron configuration is: 1s 2 2s 2 2p 6 3s 1. This
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps: The first thing you will need to do is find some information about your element. Go to the
Below is a table showing the maximum number of electrons an element can have for each of its energy level shells. The information shown is for elements with. The electronic structure of an atom
First determine what quantum numbers are indicated by the name 3s. Then note that you want the quantum numbers for an electron so m s must also be considered! Any of the following
C 2 = valence electrons =8 (σ2s) 2 (σ∗2s) 2 (σ2pz) 2 (π2px) 1 (π2py) 1 Because if the question says 2s-2p orbital intermixing is not operative it indicates you to fill the molecular orbitals
functions of many-electron atoms by applying the boundary conditions and selection rules . Pauli Exclusion Principle To understand atomic spectroscopic data for optical frequencies, Wolfgang
Question: Student Exploration: Ionic Bonds Vocabulary: chemical family, electron affinity, ion, ionic bond, metal, nonmetal, octet rule, shell, valence electron Prior Knowledge Questions (Do
From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number n and their value of l (s,
- Papa Pius Al Ii-Lea | Pius Ii Meaning
- How To Reverse Text File In Windows
- Abacus Year 1 Workbook 1
- Das Haus Am See In Byhleguhre-Byhlen 15913
- Chapitre 4: Etude De La Cavitation Dans Les Pompes
- Geschirrspüler Nachträglich Einbauen In Küchenschrank
- Deutsche Bücher – Deutsche Bücher Für Ausländer
- Bedienungsanleitung Miele Sgfk2 Complete C3 Silence Ecoline
- Want To Meet New Friends Online Apr 2024
- Flughafen Köln Bonn Handgepäck
- Uhrenwerkstatt Forum – Uhren Foren Übersicht
- El Cantante Audio Latino 1 Link