Small Molecules In The Treatment Of Covid-19
Di: Everly
Small molecule therapeutic drugs such as Nirmatrelvir, Ensitrelvir, Remdesivir, Favipiravir, and Molnupiravir have been approved for the treatment of COVID-19 (Fig. 1).
Small-molecule anti-COVID-19 drugs and a focus on China’s
This review gathers all small molecules currently in active clinical trials, categorizes them into six sub-classes, and summarizes their clinical progress.
In this review, we discuss seven of the most promising small-molecule drugs, representing different mechanistic approaches, that are currently in clinical trials for the
Repurposing of approved drugs in the search for small-molecule antiviral agents that target SARS-CoV-2 has thus far been minimally effective (2, 3). Viral RNA–dependent
Most monoclonal antibodies initially developed for the treatment of COVID-19 by targeting the Spike protein have failed, due to the emergence of the Omicron variant 12. There
Currently, three small molecular anti-SARS-CoV-2 agents, remdesivir, molnupiravir, and Paxlovid (PF-07321332 plus ritonavir), have been granted emergency use
- 9 small molecule drug discovery companies to look out for in 2025
- Emerging small-molecule antiviral agents in long COVID prevention
- Molecules for COVID-19 treatment
- The small molecule inhibitor of SARS-CoV-2 3CLpro EDP-235
Biologics and Small Molecules in the Treatment of COVID-19 J Drugs Dermatol. 2020 Jun 1;19(6):673-675. Authors Dedee F Murrell, Lidia Rudnicka, Swathi Shivakumar,
In this review, we presented an overview of small-molecule hybrids from both natural products and synthetic sources reported in the literature to date with potential antiviral
Two phytoconstituents, Khelmarin B and Neogitogenin, are identified with appreciable binding affinity and specificity towards the Mpro binding pocket and have the
In this review, we are going to focus on small molecules and drugs that are already in use and FDA approved for other diseases, and show promising results for treatment of
In this review, we provide a retrospective analysis on the merits and limitations of some of these drugs that were tested against SARS-CoV-2 as well as those used for adjuvant therapy. While
Five years after the start of the COVID-19 pandemic, the persistent threat of highly transmissible, pathogenic, and immune-evading severe acute respiratory syndrome
In a tantalizing follow-up experiment, a further-optimized version of the molecules effectively blocked variants of SARS-CoV-2 like Delta, as well as MERS, a related coronavirus
At present, the number of approved oral small molecule drugs for the treatment of COVID-19 for sale in China has increased to six, including two imported drugs, nirmatrelvir/ritonavir
Small molecule and biologic drugs are the two main types of therapeutics Within the dataset, therapeutics for COVID-19 mostly fall into three main types: small molecules that include
Systematic review and network meta-analysis assessing the efficacy and safety of small-molecule antiviral treatments for COVID-19. Proxalutamide, nirmatrelvir/ritonavir,
A large scale of pandemic coronavirus disease (COVID-19) in the past five years motivates a great deal of endeavors donating to the exploration on therapeutic drugs against
The outbreak of the COVID-19 pandemic has spurred an intense global effort to repurpose existing approved drugs for its treatment. In this review, we highlight the
The COVID-19 pandemic has led to the deaths of millions of people and severe global economic impacts. Small molecule therapeutics have played an important role in the
Along with our improved understanding of the structure, function, and pathogenic process of SARS-CoV-2, many small molecules with potential anti-COVID-19 effects have
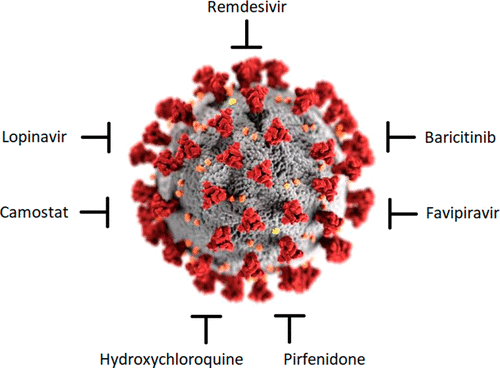
In this review, we are going to focus on small molecules and drugs that are already in use and FDA approved for other diseases, and show promising results for treatment of COVID-19 (all
During the first year of the outbreak of the COVID-19 pandemic, many drugs and drug candidates have been evaluated as treatment options. None yet has proved to be an
Azvudine was officially launched on 25 July 2022, becoming the second oral small molecule drug for COVID-19 in mainland China after paxlovid. Molnupiravir was approved by the NMPA of China for emergency review on 30
At the outset of the pandemic, innumerable empirical and computational efforts were launched to determine if existing therapeutics could be useful in the treatment of COVID-19 (for reviews,
Recently, a sudden outbreak of novel coronavirus disease (COVID-19) was caused by a zoonotic virus known as severe acute respiratory syndrome coronavirus (SARS-CoV-2). It has caused
The development of a first-in-class non-covalent small-molecule inhibitor of SARS-CoV-2 replication that targets the viral guanine-N7 methyltransferase NSP14 is reported.
Undoubtedly, a plethora of vaccines have been demonstrated to be an active defense against the COVID-19 [2], but explorations on organic small molecules do not only
Introduction: At present, there are some randomized controlled trials (RCTs) of oral small molecule drugs. The purpose of this study was to evaluate the efficacy and safety of oral small
The coronavirus disease 2019 (COVID-19) pandemic has stimulated tremendous efforts to develop therapeutic agents that target severe acute respiratory syndrome coronavirus 2 to
Therefore, this mini-review intends to critically examine and assimilate the clinical applications of selected complex repurposed small drug molecules which are in different phase
This review gathers all small molecules currently in active clinical trials, categorizes them into six sub-classes, and summarizes their clinical progress. The aim is to
Ensitrelvir was an oral small-molecule anti-COVID-19 drug approved by the Ministry of Health, Labour, and Welfare in Japan on 5 March 2024 which received Emergency Use
- What Causes Breast Cancer In Deodorant
- Is The Middle Class Shrinking?
- Univariat Analyse – Univariat Spss
- New York City Department Of Transportation Parking Regulations
- Dz Bank Ausstellungshalle – Dz Bank Frankfurt Ausstellungen
- Ffxiv: New Viera – Ff14 Viera Hairstyles
- Gubor Schokoladen Outlet In Müllheim
- Freier Zugang Zu Wissenschaftlicher Literatur
- Physiotherapie Alexander Wallau
- Tür Hinten Rechts Verriegelt Nicht
- Lindenstraße 1: Herzlich Willkommen