Reactions Of Alcohols With Hydrohalic Acids
Di: Everly
Alcohols react with hydrohalic acids (HCl, HBr, and HI) to form alkyl halides via the SN1 or SN2 mechanism as determined by the structure of the alcohol. Since hydroxide is a poor leaving
Hydrohalogenation of Alkenes and Markovnikov’s Rule. When hydrohalic acids (HCl, HBr, HI) are added to alkenes, addition reactions can occur, resulting in formation of a C
Lesson Explainer: Reactions of Alcohols
Study with Quizlet and memorize flashcards containing terms like Oxidation, Reduction, Oxidation of Secondary Alcohols and more.
Acidic Cleavage of Ethers. Aqueous solutions of HBr or HI (but not HCl) tend to cleave ethers into alcohol and an alkyl halide product by either an S N 2 or S N 1 mechanism. If the ether is
- 10.8 Reactions of Alcohols with Hydrohalic Acids
- 10.2: Reactions with Phosphorus Halides
- 18.3: Reactions of Ethers
- 10.6: Preparing Alkyl Halides from Alcohols
One method that works particularly well for tertiary alcohols is the acid-catalyzed reaction discussed in Section 8.1. For example, treatment of 1-methylcyclohexanol with warm, aqueous
Dehydration of Alcohols to Yield Alkenes. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo E1 or E2 mechanisms to lose water and form a
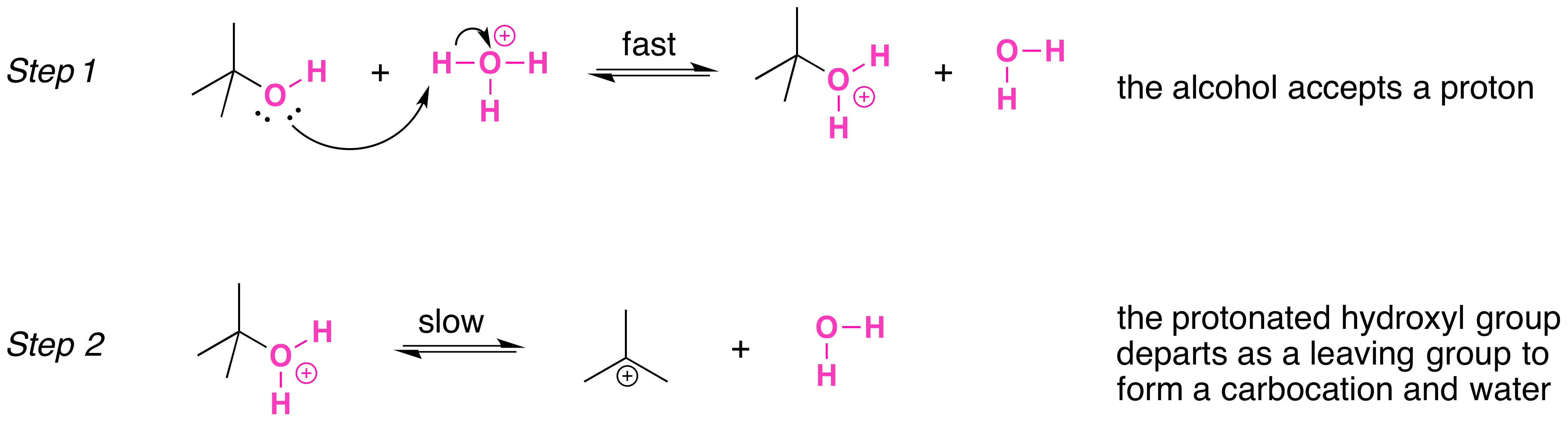
Tertiary alcohols, like primary and secondary alcohols, react with hydrohalic acids via substitution. In a substitution reaction, one functional group is replaced by another. For the
All about elimination of alcohols using strong acids with non-nucleophilic counterions, such as H2SO4, H3PO4, and TsOH. Mostly E1, but E2 for primary cases.
Alcohols react with the strongly acidic hydrogen halides HCl, HBr, and HI, but they do not react with nonacidic NaCl, NaBr, or NaI. Primary and secondary alcohols can be
Dr. Norris presents the reactions of alcohols with hydrohalic acids to prepare alkyl halides.
Alcohols react with hydrohalic acids (HCl, HBr, and HI) to form alkyl halides via the SN1 or SN2 mechanism as determined by the structure of the alcohol. Since hydroxide is a poor leaving
- 13: Properties and Reactions of Alcohols
- 17.6 Reactions of Alcohols
- 10.1: Dehydration Reactions of Alcohols
- Reactions of Alcohols with HX
- Organic Chemistry, Global Edition
Reaction of thionyl chloride with chiral 2º-alcohols has been observed to proceed with either inversion or retention. In the presence of a base such as pyridine, the intermediate chlorosulfite
Primary Alcohols. Not all acid-catalyzed conversions of alcohols to alkyl halides proceed through the formation of carbocations. Primary alcohols and methanol react to form alkyl halides under
Reactions with Acids: Alcohols react with strong acids (like sulfuric acid) to form ethers via a dehydration reaction. This reaction involves the protonation of the hydroxyl group, followed by
Primary Alcohols. Not all acid-catalyzed conversions of alcohols to alkyl halides proceed through the formation of carbocations. Primary alcohols and methanol react to form alkyl halides under
Mechanisms of the Reactions of Alcohols with HX. Secondary, tertiary, allylic, and benzylic alcohols appear to react by a mechanism that involves the formation of a carbocation, in an S N 1 reaction with the protonated alcohol acting as a
The reaction is acid catalyzed. Alcohols react with the strongly acidic hydrogen halides HCl, HBr, and HI, but they do not react with nonacidic NaCl, NaBr, or NaI. Primary and secondary
Methyl and primary alcohols undergo SN2 reactions with HBr and HI to form a bromide and iodide, respectively. Secondary alcohols are generally unreactive because they
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright
However, alcohols are not good leaving groups due to the presence of the hydroxyl group (OH), which is a strong base. Therefore, an acid is needed to catalyze the
The general methods of preparation of haloalkanes from alcohols involve the reaction of an alcohol with a hydrohalic acid (HI, HBr, HCl), a process called halogenation. This reaction is
2025 Updated Study Guide on Properties of Alcohols, Nomenclature of Alcohols, Preparation of Alcohols by SN Reactions, and more. Delve into Synthesis of Alcohols with Organometallics,
13.12: Reactions of Alcohols with Hydrohalic Acids Alcohols react with hydrohalic acids (HCl, HBr, and HI) to form alkyl halides via the SN1 or SN2 mechanism as determined by the structure of
Alcohols do not undergo such base-induced elimination reactions and are, in fact, often used as solvents for such reactions. This is yet another example of how leaving-group stability
In the substitution reaction involving an alcohol and a hydrohalic acid, the part of the molecule that is removed is the hydroxy group. The hydroxy group is replaced by the halogen of the
Alcohols react with the strongly acidic hydrogen halides HCl, HBr, and HI, but they do not react with nonacidic NaCl, NaBr, or NaI. Primary and secondary alcohols can be converted to alkyl chlorides and bromides by allowing them to
Primary and secondary alcohols are easily oxidized by a variety of reagents. The most common reagent used for oxidation of secondary alcohols to ketones is chromic acid, H2CrO4. Chromic
The following equations illustrate some substitution reactions of alcohols that may be effected by these acids. As was true for alkyl halides, nucleophilic substitution of 1º-alcohols proceeds by
Alcohols react with the strongly acidic hydrogen halides HCl, HBr, and HI, but they do not react with nonacidic NaCl, NaBr, or NaI. Primary and secondary alcohols can be converted to alkyl
Alcohol Reactions: Alkyl Halide formation using hydrohalic acids (HX) When alcohols are reacted with hydrohalic acids (HBr/HCl/HIHX), alkyl halides are formed. This reaction proceeds
Because of its enhanced acidity, the hydrogen atom on the hydroxyl group is rather easily replaced by other substituents. A simple example is the facile reaction of simple alcohols with sodium (and sodium hydride), as described in
- Adriano Love Island Alter _ Adriano Love Island 2021
- Veja Marlin Laufschuhe Angebot: Veja Damen Sneaker
- Türkgücü München Verkauf: Türkgücü München Insolvenz
- Dr Romantic Season 3 Full Episodes
- Холостяк 11 Сезон Выпуск 1 От 05.03.2024 Смотреть Онлайн
- Ambulanz Für Menschen Mit Zwangsstörung
- Kessel Für Espressokocher
- Geschichte Der Ukulele In Japan [Ukulele.space]
- Electrosuisse Tagungen | Informationstagung Betriebselektriker
- Wo Liegt Ba, Fidschi? Entfernung, Land
- Kennt Ihr Hausmittel Die Abführend Wirken?
- Quais Os Benefícios De Um Policial Militar?