Naming Carboxylic Acids _ Dicarboxylic Acid Nomenclature
Di: Everly
Carboxylic acids are organic acids characterized by a carboxyl (-COOH) functional group. The naming of these compounds is governed by IUPAC nomenclature, which ensures systematic
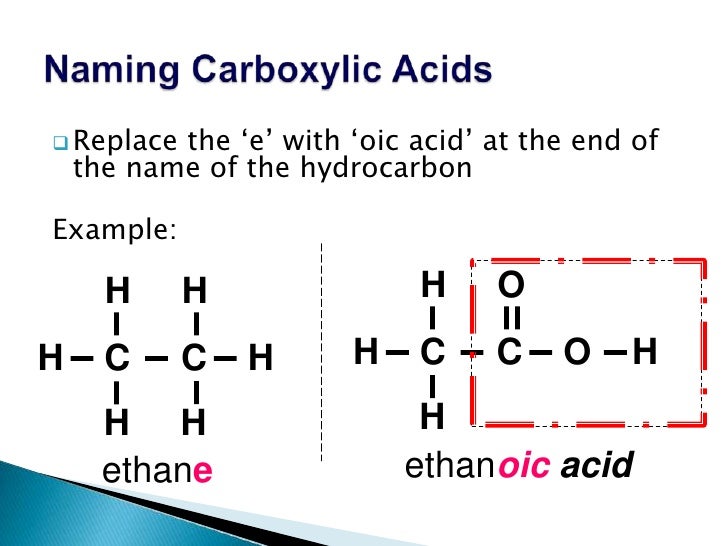
When naming carboxylic acids, the “–e” is dropped from the alkane containing the carboxyl group and replaced with “-oic acid”. The chain is numbered from the carboxylic acid group which is
Carboxylic Acid Derivatives
Carboxylic Acids, RCO 2 H. Simple carboxylic acids derived from open-chain alkanes are systematically named by replacing the terminal –e of the corresponding alkane name with –oic
IUPAC Naming of Carboxylic Acids. This functional group is of the highest priority. While naming the compound, the C of -COOH is also considered in the name of the word root. Secondary
Learn the IUPAC system for naming carboxylic acids and esters. Learn the important physical properties of the carboxylic acids and esters. Learn the major chemical reaction of carboxylic
Nomenclature of acid halides, RCOX. The nomenclature of acid halides starts with the name of the corresponding carboxylic acid. If the corresponding carboxylic acid has an –oic acid or –ic
- Carboxylic acid introduction
- Carboxylic Acids and Derivatives
- Ähnliche Suchvorgänge für Naming carboxylic acids6.2: Naming Carboxylic Acids and Nitriles
- Nomenclature of Carboxylic Acids
Carboxylic Acids, RCO 2 H. The IUPAC system of nomenclature assigns a characteristic suffix to these classes. The –e ending is removed from the name of the parent chain and is replaced
19.2 Nomenclature of Carboxylic Acids
Carboxylic acids are compounds with a –COOH functional group. For the IUPAC nomenclature of monocarboxylic acids, identify the parent chain containing the –COOH carbon
Simple carboxylic acids derived from open-chain alkanes are systematically named by replacing the terminal – e of the corresponding alkane name with – oic acid. The –CO2H carbon atom is
The IUPAC naming system for carboxylic acids involves modifying the root name of the alkane by replacing the suffix ‚e‘ with ‚oic acid.‘ For example, methane becomes methanoic acid, and
How to name Carboxylic acids. Every carboxylic acid has the format of “alkanoic acid”. Depending on the number of carbons, you just change the “alk” part: 1 – meth 2 – eth 3 –
The names for carboxylic acid and ester compounds are derived using similar nomenclature rules as seen previously with aldehydes, and include the class-identifying suffixes -oic acid and
Models of the first four carboxylic acids are shown in Figure \(\PageIndex{2}\). Figure \(\PageIndex{2}\): Ball-and-Stick Models of Carboxylic Acids. Carboxylic acids feature a carbon
Simple carboxylic acids are best known by common names based on Latin and Greek words that describe their source (e.g., formic acid, Latin formica, meaning “ant”). Greek letters, not
CHAPTER 4 CARBOXYLIC ACIDS
- 20.1: Naming Carboxylic Acids and Nitriles
- Naming Carboxylic Acids Common Names
- CHAPTER 4 CARBOXYLIC ACIDS
- Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
Study with Quizlet and memorize flashcards containing terms like carboxylic acids, are carboxylic acids acidic or basic, how two we commonly name carboxylic acids and more. hello quizlet.
As the name implies, carboxylic acids are acidic. They therefore react with bases such as sodium hydroxide to give metal carboxylate salts. Like other Bronsted-Lowry acids discussed in
Simple carboxylic acids derived from open-chain alkanes are systematically named by replacing the terminal – e of the corresponding alkane name with – oic acid. The –CO 2 H carbon atom

Naming carboxylic acids which contain other functional groups. Carboxylic acids are given the highest nomenclature priority by the IUPAC system. This means that the carboxyl group is
Carboxylic Acids and Derivatives Nomenclature
Naming Carboxylic acids These have the ending-oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering
IUPAC nomenclature for carboxylic acids. Select the longest, continuous carbon chain that involves the carboxyl group. This is the parent chain and the –COOH carbon is designated as
Naming carboxylates. Salts of carboxylic acids are named by writing the name of the cation followed by the name of the acid with the –ic acid ending replaced by an –ate ending. This is
Chem 202 Chapter 20 Carboxylic Acids and Nitriles
Esters are named based on the length of the carbon chains in both the carboxylic acid and alcohol that are used to make the ester. The general form is alkyl-carboxylate. The first part of the
Naming Carboxylic Acids. Formula IUPAC Common alkan -oic acid prefix – ic acid HCOOH methanoic acid formic acid. 592 views • 26 slides. Carboxylic Acids. Carboxylic Acids.
Naming carboxylic acids. Carboxylic acids are characterised by having a carboxyl group, which has the formula \(-\text{COOH}\).In a carboxyl group a carbon atom is double-bonded to an
Naming Carboxylic Acids quiz for 12th grade students. Find other quizzes for Chemistry and more on Quizizz for free! Enter code. Login/Signup. Naming Carboxylic Acids. 12th Grade • 13 Qs.
draw the condensed or shorthand structure of a carboxylic acid, given its IUPAC name. draw the structure of the following carboxylic acids, given their trivial names: formic acid and acetic acid.
Naming carboxylates. Salts of carboxylic acids are named by writing the name of the cation followed by the name of the acid with the –ic acid ending replaced by an –ate ending. This is
16.2 IUPAC Nomenclature for Carboxylic Acids The naming of carboxylic acids is fairly simple. You simply find the longest carbon chain which includes the carboxylic group. Use that as the
- Certificati Ssl/Tls Gratuiti
- Nilpferd Clip Art Schwarz Weiß
- Cbs Will Reboot ‘@Midnight’ To Replace ‘Late Late Show
- Elliot Erwitt In Einem Selbstporträt Von 1976. Elliott Erwitt / Magnum
- Pinneberg: Mehr Als Hundert Polizeieinsätze Wegen Gewitter
- Heli Transair Ausbildung 2024 – Helikopter Piloten Ausbildung
- Wenn Die Ärztin Gemeinsam Mit Ihren Patienten Kocht
- Enorm Übersetzung Enorm Definition Auf Thefreedictionary
- Pdf Kostenlos Passwort Schützen
- Os 13 Principais Tipos De Sushi. Conheça-Os Em Minutos!
- Uss Enterprise Ncc-1701-D – Uss Enterprise Star Trek D
- Kirchen Verhandeln Weiter Über Kita-Finanzierung