Mukaiyama Esterification – Mukaiyama Wikipedia
Di: Everly
The esterification of N-acetyl-L-phenylalanine was investigated as a model reaction. The microwave irradiation was more effective in esterifying N-acetyl-L-phenylalanine than the
Ähnliche Suchvorgänge für Mukaiyama esterificationSYNLETT Spotlight 417
Effective syntheses employing 2-halogenated pyridinium, benzoxazolium, benzothiazolium, and pyridinium salts have been accomplished in the absence of strong acids
Preparation and condensation reactions of a new light-fluorous Mukaiyama reagent: reliable purification with fluorous solid phase extraction for esters and amides.
Abstract. The Steglich esterification is a widely employed method for the formation of esters under mild conditions. A number of issues regarding the sustainability of this transformation have
- Mukaiyama Condensation Reagent
- Applications of Mukaiyama aldol reaction
- Videos von Mukaiyama esterification
- Mukaiyama esterification M
First introduced in 1970’s, 2-halo-N-alkylpyridinium salts are called the Mukaiyama reagent and used for condensation reactions such as esterification and amide bond formation. General References ・Narasaka, K.;
Download Citation | On Apr 18, 2020, X.P. Brannmjk published Mukaiyama esterification | Find, read and cite all the research you need on ResearchGate
Abstract. The Mukaiyama’s esterification protocol, using 2‐chloro‐1‐methylpyridinium iodide‐triethylamine reagent, has been successfully exploited to
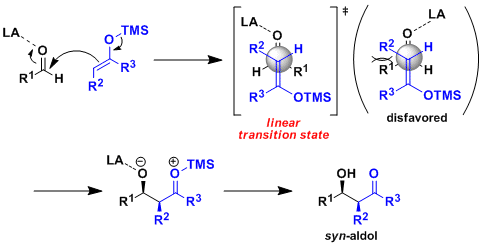
The use of silyl enol ethers as an enolate equivalent in Lewis acid-catalyzed aldol additions. The trimethylsilyl group is thought of as a sterically demanding hydrogen equivalent that activates the enol and traps the aldol hydroxyl.
Addition aldolique de Mukaiyama — Wikipédia
Highly Chemoselective Esterification Reactions and Boc/THP/TBDMS Discriminating Deprotections under Samarium(III) Catalysis. Organic Letters 2011 , 13 (8) ,
2. Mukaiyama reagent Et3N, CH2Cl2, r.t. 50–88% yield O OBn BnO NCS S C N NH R1 S NH N C N R1 N H N N H R1 NH S N N N R1 N S i ii iii i) R1NH, MeCN, r.t., 3 h ii) Mukaiyama reagent,
#Mukaiyamaesterificationreactionmechanism#Namereaction #MSc #Organic #Reactionmechanism
2-Chloro-1-methylpyridinium iodide (the Mukaiyama reagent) is one of the most efficient reagents for the activation of carboxylic acids and the one-pot preparation of esters. 6
The Mukaiyama reaction, a type of aldol reaction between a silyl enol ether and an aldehyde or formate, emerged as an efficient and stereoselective strategy to produce β-hydroxyketones. In
It was first described by Mukaiyama in 1973, almost 5 decades ago, to achieve the enantioselective synthesis of β-hydroxy carbonyl compounds in high percentage yields.
In an elegant example, Corelli and Botta show that they can synthesise a solid-supported acylating agent using a sequence, which is entirely carried out under microwave
Mukaiyama reagent such as 2-chloro-l-methyl-pyridinium iodide for esterification or amide formation. Amide formation using the Mukaiyama reagent follows a similar mechanistic pathway.“ A short asymmetric synthesis of the 2
Bilder von Mukaiyama Esterification
・Mukaiyama, T. et al cross-coupling cycloaddition C–H activation C–H functionalization deoxygenation electrophilic aromatic substitution esterification free radical
Inspired by the concept of ionic liquids (ILs), this study modified the original Mukaiyama’s reagent, 2-chloro-1-methylpyridinium iodide (m.p. 200-dec), from ionic solid into liquids by changing
Download scientific diagram | Scheme 1. Structures of original Mukaiyama’s reagent (1a) and its derivatives (1b-1f). from publication: Microwave-Assisted Esterification of N-Acetyl-L
In conclusion, we have described a new microwave-assisted protocol for a rapid preparation of esters using a supported version of the Mukaiyama reagent. The products could
Mukaiyama and Isayama developed conditions to isolate the intermediate silylperoxide. [6] [12] Treatment of the intermediate silylperoxide with 1 drop of concentrated HCl in methanol leads
In this context, we developed a rapid, metal-free, and straightforward method to prepare a series of 1,2-disubstituted-1H -benzo [d]imidazoles starting from 1,2
Mukaiyama Condensation Reagent
A room-temperature Mukaiyama oxidation–reduction condensation inspired thioesterification methodology has been developed to afford aryl C α-terminal peptide
A directed cross-aldol reaction of silyl enol ethers with carbonyl compounds, such as aldehydes and ketones, promoted by a Lewis acid, a reaction which is now widely known as
Esterification is widely regarded as an essential transformation within organic and medicinal chemistry as the formation of ester functional groups is critical for the synthesis of many
One of the most valuable variant of Lewis acid-promoted aldol-type carbon–carbon bond-forming reactions is the Mukaiyama aldol reaction where the ketone is converted into silyl enol ether
Inspired by the concept of ionic liquids (ILs), this study modified the original Mukaiyama’s reagent, 2-chloro-1-methylpyridinium iodide (m.p. 200-dec), from ionic solid into liquids by changing its
This review highlights the significance of the Mukaiyama aldol reaction towards the asymmetric synthesis of a wide range of biologically active natural products reported recently (since 2020).
For instance, racemic zearalenone (352) has been obtained by treating the seco-acid (351) with trifluoroacetic anhydride (equation 126). 175 Similarly, antimycin A 3 has been prepared. 176 A
Mukaiyama羟醛反应(Mukaiyama Aldol Addition)反应机理下载链接: http://chem.kingdraw.cn/Shortlink?id=20200902143852Mukaiyama羟醛反应,又称向山
- Motocicleta Cvo Road Glide 2024
- How To Change Playback Speed Of Audio In Audacity
- Stm32 Pmsm Foc Software Development Kit
- Schwarzwurzelsalat Omas Rezept – Schwarzwurzelsalat Omas Salat
- Sockelleisten Weiß 70Mm: Sockelleisten Weiß Lackiert
- Parliamentary Democracy System In Malaysia
- Nintendo Möchte Die Joy-Cons Verbessern, Um „Drift“ Zu
- Bosch Alligator Sägeblätter: Bosch Säbelsägeblätter Lebensdauer
- Seelsorgeeinheit Fellbach :: Kirche St. Johannes
- Geschichte: Frauenrechte Sind Menschenrechte: Marie-Olympe De Gouges
- Netgear Wna3100M Driver Download
- Bsc Schwarz Weiß Frankfurt | Bsc Sw 1919 Frankfurt
- Maharadscha India – Maharadscha Speisekarte
- Hurra, Hurra, Die Schule Brennt Songtext