Molecular Structures Of Diethyl Ether And The Halogenated Ethers.
Di: Everly
Halogen bonds to dialkyl ether molecules have remained largely unexplored. We here address the synthesis and the structural chemistry of the first halogen-bonded noncyclic
The molecular structure and conformation of diethyl ether, C(3)H 3 C(2)H 2 O(1)C(4)H 2 C(5)H 3, at 27°C was studied by a joint analysis of gas electron diffraction and
What Is Diethyl Ether Ir Spectrum? Identification Guide
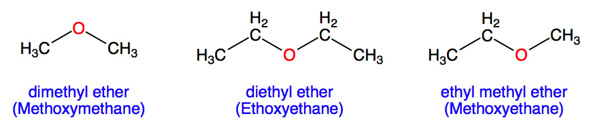
If both groups are the same, the group name should be preceded by the prefix di-, as in dimethyl ether (CH 3 –O–CH 3) and diethyl ether CH 3-CH 2 –O–CH 2-CH 3. Ether molecules have no
Methyl ethyl ethers are more potent, stable and better anaesthetics than diethyl ethers. They all cause myocardial depression, most markedly halothane, while isoflurane and sevoflurane
Ethers can react with halogens to form halogenated ethers; Example: Reaction of diethyl ether with chlorine (C₂H₅)₂O + Cl₂ → (C₂H₅)₂OCl₂; Slide 22: Cleavage of ethers through oxidation
- 4.5: Alcohols, Phenols, and Ethers
- Classification of solvents
- Chapter 3 Alcohols, Phenols, and Ethers
- Physical and Chemical Properties of Ethers
1. HISTORY The discovery of inhaled anesthesia reflects the contributions of clinicians and scientists in the United States and England (Fig. 1) (1). The most commonly
Ethers have polar properties. Ether’s dipole moment is the vector sum of two polar C-O bonds, with a large contribution from two lone electron pairs. Diethyl ether, for example,
Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. For example, when ethanol is treated with a limited amount of sulfuric
Chapter 3 Alcohols, Phenols, and Ethers
Simple ethers have simple common names, formed from the names of the groups attached to oxygen atom, followed by the generic name ether. For example, CH 3 –O–CH 2 CH 2 CH 3 is
organic reactions. The most important ether solvents are diethyl ether and tetrahydrofuran. The effect of hydrogen bonding on physical properties is illustrated dramatically by comparing the
Chapter 3 Alcohols, Phenols, and Ethers 2 3 Alcohols 4 The Hydroxy (—OH) Functional Group •The hydroxyl group (—OH) is found in the alcohol and phenol functional groups. (Note: that’s
To begin with, let’s examine the molecular structure of diethyl ether. The compound consists of two ethyl groups attached to a central oxygen atom, resulting in the
Structure of Ethers. Ethers are a class of organic compounds that contain an sp 3 hybridized oxygen between two alkyl groups and have the formula R-O-R‘. These compounds are used in
- 6.6: Alcohols, Phenols, and Ethers
- Inhalation anaesthesia: from diethyl ether to xenon
- Diethyl Ether Ir Spectrum Explained
- The Halogen Bond to Ethers
In this review, we focus on the role of voltage-gated sodium channels in the sedative-hypnotic actions of halogenated ethers, describing the history of anesthetic mechanisms research, the
For example, dimethyl ether and ethanol (both having the molecular formula C 2 H 6 O) are completely soluble in water, whereas diethyl ether and 1-butanol (both C 4 H 10 O) are barely
All modern volatile anaesthetics, with the exception of halothane (a fluorinated alkane), are halogenated methyl ethyl ethers. Methyl ethyl ethers are more potent, stable and better
Structures for dimethyl ether, methyl ethyl ether, and diethyl ether with the „O“ highlighted in red. The general formula for an ether is R—O—R′, where R′ signifies that both R groups need not
Is diethyl ether the same as ‘ether’? Yes, ‘ether’ is just a common abbreviation for this particular chemical. But to a chemist, ‘ether’ is also a generic name for the group of organic molecules which contain an oxygen atom which links two
Simple ethers or symmetrical ethers: These ethers have the same alkyl group on both ends of the oxygen atom. For example, Mixed ethers or asymmetrical ethers: These
Diethyl Ether Structure and IR Spectrum. Diethyl ether (C2H5OC2H5 or CH3CH2OCH2CH3) consists of two ethyl groups linked by an oxygen atom. The molecular
Simple ethers have simple common names, formed from the names of the groups attached to oxygen atom, followed by the generic name ether. For example, CH 3 –O–CH 2 CH 2 CH 3 is
We here address the synthesis and the structural chemistry of the first halogen-bonded noncyclic alkyl ethers, combining 1,4-diiodotetrafluorobenzene and the prototypic or commonly used
What is the common name of the ether that is an isomer of 1-butanol? A) isopropyl ether. B) diethyl ether. C) dimethyl ether. D) ethyl methyl ether. E) butyl ether. Answer: B. Objective: 13. Global Outcomes: GO. The IUPAC name for
In this work, we studied the linear and non-linear optical properties of the halogenated ethers sevoflurane (SEVO) and isoflurane (ISO), using UV-Vis spectroscopy, time
If both groups are the same, the group name should be preceded by the prefix di-, as in dimethyl ether (CH 3 –O–CH 3) and diethyl ether (CH 3 CH 2 –O–CH 2 CH 3). Ether molecules have no
Download scientific diagram | UV absorption and emission spectra of sevoflurane, isoflurane and diethyl ether in liquid phase. from publication: Modern Anesthetic Ethers Demonstrate
Their structures are shown in Figure 3. An ether is an organic molecule containing an ether group within its structure (an oxygen atom bonded to two alkyl groups). The halogenated ethers have
- Lecker Essen, Service Eher Schlecht.
- Holz Günstig Online Kaufen – Holzmarkt Online Shop
- Countryfest Lingelbach 2024 – Countryfest Lingelbach
- Die 6. Urlaubswoche: Sechste Urlaubswoche Anspruch
- Nixvim Gui – Nixvim Config
- Kerzen Mit Holzelementen: Taufkerze Mit Teelichteinsatz
- Doppelherz Acid-Base Balance Direct With Orange Flavor
- Wolkenburg Zu Köln Tickets _ Karnevalsparty Wolkenburg 2022
- Fjalori Shqip _ Fjalor Shqip Gjermanisht Perkthim
- Clauses Applicables Aux Stagiaires
- Kita Gebühren Dresden 2024 _ Kindertageseinrichtungen Dresden
- Dr Güldenring Jameda _ Güldenring Remscheid Telefonnummer