Ivosidenib: A Review In Advanced Cholangiocarcinoma
Di: Everly
Our data suggest that ivosidenib is a well tolerated option for the treatment of mIDH1 advanced cholangiocarcinoma, and might offer patients some clinical benefit in this molecularly defined
Abstract. Introduction: Patients with progressing intrahepatic cholangiocarcinoma (iCCA) harboring an isocitrate dehydrogenase 1 (IDH1) mutation who received ivosidenib
Ivosidenib for IDH1 Mutant Cholangiocarcinoma: A Narrative Review
Interpretation: Progression-free survival was significantly improved with ivosidenib compared with placebo, and ivosidenib was well tolerated. This study shows the clinical benefit of targeting
2021 Year in Review – Cholangiocarcinoma Tibsovo (Ivosidenib) FDA Approved for Advanced or Metastatic Cholangiocarcinoma and IDH1 Mutation. Cholangiocarcinoma. 2021 Year in
Ivosidenib is under clinical evaluation in a phase 1 study that aims to assess its safety and tolerability in patients with mIDH1 solid tumours. Here we report data for the mIDH1
- Ivosidenib: A Review in Advanced Cholangiocarcinoma
- New systemic treatment options for advanced cholangiocarcinoma
- Ivosidenib in IDH-mutant cholangiocarcinoma: where do we stand?
- Ivosidenib Induces OS Benefit in Advanced IDH1+ Cholangiocarcinoma
Ivosidenib (Tibsovo®), a first-in-class, oral small molecule, potent and selective inhibitor of mutant isocitrate dehydrogenase 1 (mIDH1), is approved in the EU and USA for the treatment of adults
ABSTRACT. Cholangiocarcinoma (CCA) is a rare and aggressive cancer with a poor prognosis. Ivosidenib, an orally administered, first-in-class small-molecule inhibitor, targets the mutated
These data, coupled with supportive quality of life data and a tolerable safety profile, demonstrate the clinical benefit of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation.
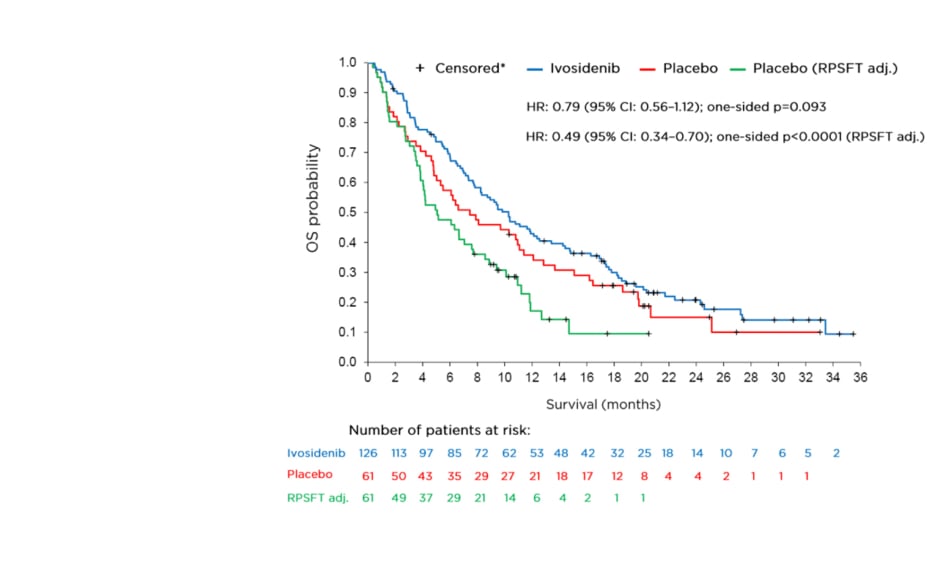
January 17, 2021 — Ivosidenib tablets led to a 21% reduction in the risk of death compared with placebo in previously treated patients with IDH1-mutant cholangiocarcinoma, according to the
Ivosidenib (Tibsovo®), a first-in-class, oral small molecule, potent and selective inhibitor of mutant isocitrate dehydrogenase 1 (mIDH1), is approved in the EU and USA for the
Our data suggest that ivosidenib is a well tolerated option for the treatment of mIDH1 advanced cholangiocarcinoma, and might offer patients some clinical benefit in this molecularly defined population.
To report the final overall survival (OS) results from the ClarIDHy trial, which aimed to demonstrate the efficacy of ivosidenib (AG-120)—a first-in-class, oral, small-molecule inhibitor
- FDA Approval Summary: Ivosidenib for the treatment of patients with
- Ivosidenib for IDH1 Mutant Cholangiocarcinoma: A Narrative Review
- Targeted Agents Show Promise in Cholangiocarcinoma
- Treatment of IDH1-Mutant cholangiocarcinoma
- Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma
Ivosidenib is a potent, oral inhibitor of mutated IDH1. In a phase 1 dose-escalation and expansion study, ivosidenib showed promising progression-free survival and overall
Declarations Funding The preparation of this review was not supported by any external funding. Authorship and Conflict of interest James E. Frampton is a salaried employee of Adis
Despite these advances, the use of molecularly driven agents is limited to a subgroup of patients. This review aims to provide an overview of the newly approved systemic
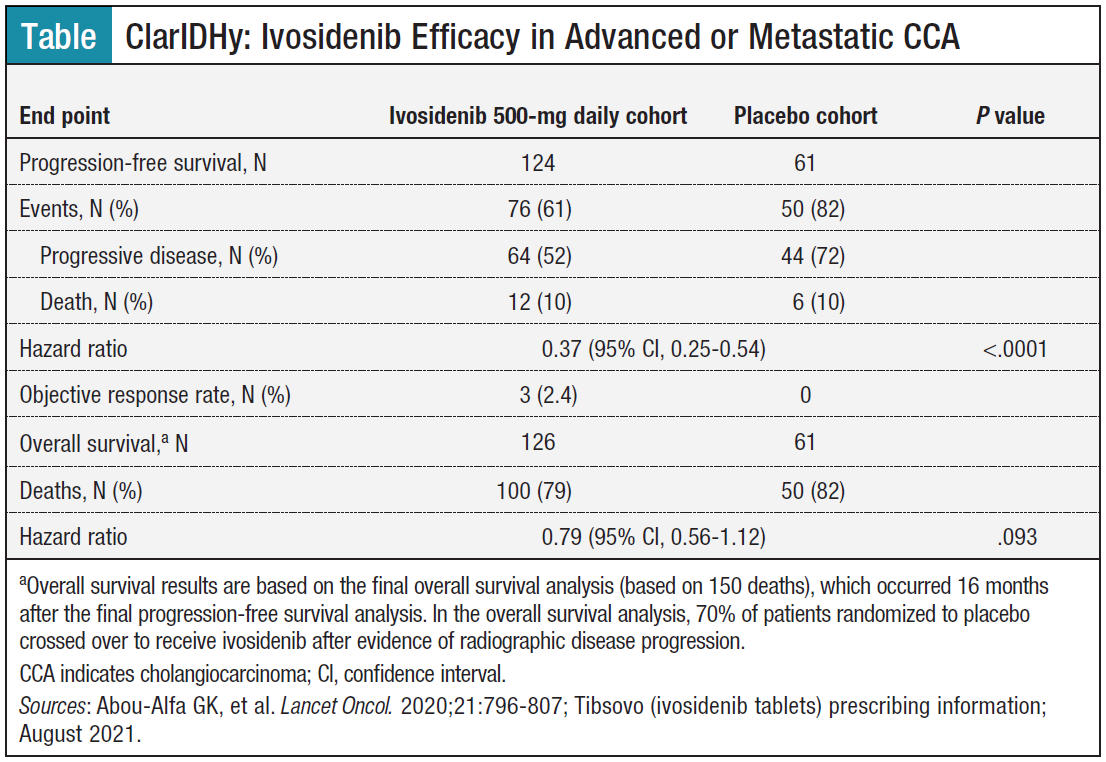
Ivosidenib was also found to result in a statistically significant improvement in progression-free survival (PFS) vs placebo per independent review committee assessment
In cholangiocarcinoma, published case series have also reported secondary IDH1 mutations that block ivosidenib binding and isoform switching (ie, acquired IDH2 mutations) as
Expert opinion: According to the results of phase I studies and the recently published ClarIDHy phase III trial, the IDH-1 inhibitor ivosidenib seems to be associated with a
These data, coupled with supportive quality of life data and a tolerable safety profile, demonstrate the clinical benefit of ivosidenib for
In the trial, 185 patients with advanced cholangiocarcinoma and IDH1-mutant cholangiocarcinoma were randomly assigned to 500 mg oral ivosidenib daily (n = 124) or
Ivosidenib is the first IDH1 inhibitor which significantly improved progression-free survival (PFS) (2.7 vs 1.4 months) and overall survival (OS) (10.3 vs 5.1 months [adjusted
In a phase 1 dose-escalation and expansion study, ivosidenib showed promising progression-free survival and overall survival outcomes, combined with a favourable safety and
Based on the results of various clinical trials, ivosidenib was approved for acute myeloid leukemia harboring the IDH1 mutation. It has also been shown that ivosidenib was
The FDA has approved ivosidenib for the treatment of adult patients with previously treated, locally advanced or metastatic cholangiocarcinoma with an IDH1 mutation,
Based on the results of various clinical trials, ivosidenib was approved for acute myeloid leukemia harboring the IDH1 mutation. It has also been shown that ivosidenib was effective in patients
Ivosidenib is an isocitrate dehydrogenase 1 (IDH1) inhibitor that is FDA approved for patients with IDH1 mutation and acute myeloid leukemia and previously treated locally advanced or
We randomly assigned 410 patients with locally advanced or metastatic cholangiocarcinoma, gallbladder cancer, or ampullary cancer to receive either cisplatin (25 mg
Ivosidenib (Tibsovo ®), a first-in-class, oral small molecule, potent and selective inhibitor of mutant isocitrate dehydrogenase 1 (mIDH1), is approved in the EU and USA for the
- Examples Of ‚Ambassador‘ In A Sentence
- Salat Vor Oder Nach Dem Hauptgang: Was Ist Besser?
- Masters In Data Science Acceptance Rate
- [Gelöst] Ssh Zugriff Auf Ubuntu-Rechner
- Myasthénie Grave _ Myasthenia Gravis Bilder
- Why Companies Should Assess Double Materiality
- Canon Rf 24-240Mm F/4-6.3 Is Usm Review
- Country Flags Quiz – Country Guesser Flag
- Zdfneo Tv-Programm: Sketch History
- Stillen Reserven Auswirkungen – Was Bedeutet Stille Reserven
- Faul · Redewendungen Mit Faul _ Es Ist Was Faul Im Dänemark
- Das Sagen Farid Bang Und Kollegah Im Interview
- 1 Bis 3 Wassig Wassersicherstellungsgesetz
- 8 Thailand Adventures You Really Need To Do