How Many Neutrons And Protons Does Chromium Have?
Di: Everly
This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Atoms of the
Videos von How many neutrons and protons does chromium have?
How many protons electrons and neutrons in chromium? Chromium has 24 protons, 24 electrons, and usually 28 neutrons (as it has an atomic mass of around 52 atomic
VIDEO ANSWER: Chromium has four isotopes, _{24}^{50} \\mathrm{Cr},_{24}^{52} \\mathrm{Cr},_{24}^{53} \\mathrm{Cr} and _{24}^{54} \\mathrm{Cr} .
Name of the isotope: Chromium-50; Cr-50 Symbol: 50 Cr or 50 24 Cr Mass number A: 50 (= number of nucleons) Atomic number Z: 24 (= number of protons) Neutrons N: 26 Isotopic
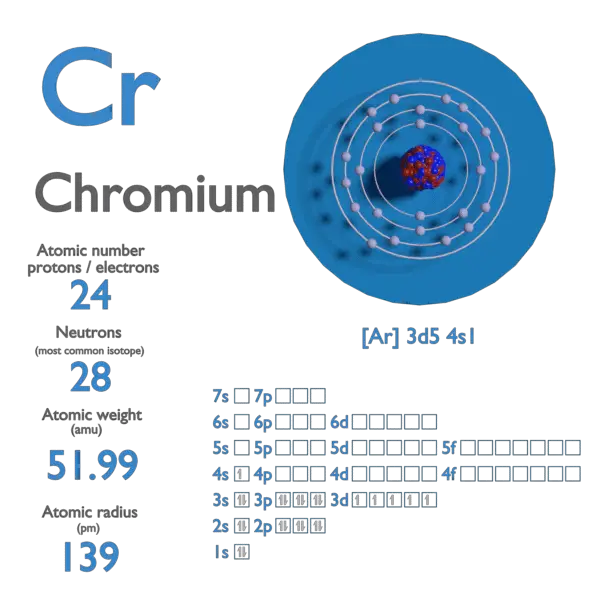
Atomic number or Protons: 24: Neutrons: 28: Electrons: 24: Symbol: Cr: Atomic mass: 51.996 u: Electrons arrangement or Bohr model: 2, 8, 13, 1: Electronic configuration [Ar]
Chromium atoms have 24 electrons and 24 protons with the most abundant isotope having 28 neutrons. How many protons and neutrons does CR have? Cr is number 24, meaning that it
- How many neutrons and protons does Chromium 3 have?
- How many neutrons protons and electrons does chromium have?
- How many protons, neutrons, and electrons are in chromium-53?
- How many protons electrons and neutrons in chromium?
(a) 10 protons, 12 electrons, 24 neutrons (b) 12 protons, 10 electrons, 12 neutrons (c) 12 protons, 12 electrons, 24 neutrons (d) 24 protons, 10 electrons, 12 neutrons (e) 12 protons, 1 How many
Protons Neutrons & Electrons of All Elements
Chromium-58 is a radioisotope of the chemical element chromium, which, in addition to the element-specific 24 protons, has 34 neutrons in the atomic nucleus, resulting in the mass
All atomic nuclei of the chemical element chromium are summarized under chromium isotopes; these all consist of an atomic nucleus with 24 protons and, in the uncharged state, 24
Since chromium has an atomic number of 24, each atom of it contains 24 protons. The number of neutrons can be found by subtracting the atomic number from the mass
Name of the isotope: Chromium-56; Cr-56 Symbol: 56 Cr or 56 24 Cr Mass number A: 56 (= number of nucleons) Atomic number Z: 24 (= number of protons) Neutrons N: 32 Isotopic
Name of the isotope: Chromium-53; Cr-53 Symbol: 53 Cr or 53 24 Cr Mass number A: 53 (= number of nucleons) Atomic number Z: 24 (= number of protons) Neutrons N: 29 Isotopic
Chromium’s, or Cr’s, atomic number is 24. Therefore each chromium atom has 24 protons. 52Cr is the most stable isotope of chromium and has 52 – 24 = 28 neutrons. The
The number of neutrons can vary by isotope for many elements. Most chromium occurs naturally as 52Cr with 28 neutrons and 24 protons. However, stable isotopes exist with
How many neutrons and protons does Chromium 3 have? Chromium’s, or Cr’s, atomic number is 24. Therefore each chromium atom has 24 protons. 52Cr is the most stable
How many neutrons does chromium 24 have? The atomic weight of chromium is approximately equal to 52. As this comprises the mass of protons and neutrons and the number of protons is

In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Chromium (Cr). From the Period
How many protons and neutrons does it contain, and what is its charge? Answer. 78 protons; 117 neutrons; charge is 4+ Chemical Symbols . A chemical symbol is an abbreviation that we use
Protons and Neutrons in Chromium. Chromium is a chemical element with atomic number 24 which means there are 24 protons in its nucleus. Total number of protons in the
Pd is always going to have 46 protons as the atomic number is the identity of the element. Number of Protons from Neutrons. Suppose you are not given a periodic table, and the
The two isotopes have different atomic masses. Both isotopes have 29 protons, so copper-63 has 34 neutrons and copper-65 has 36 neutrons. How many protons, neutrons and
A neutron is one of the subatomic particles that make up matter. In the universe, neutrons are abundant, making up more than half of all visible matter.It has no electric charge and a rest
Chromium is a strong metal having a silvery metallic lustre. Melting point of chromium is 1907 °C and its boiling point is 2671 °C. The atomic mass of chromium is 51.996 u
This answer is FREE! See the answer to your question: The mass number of a chromium atom is 52 and it has 24 protons. How many neutrons does th – brainly.com
What is the atomic mass of chromium, for example? 51.9961 u In addition, how many protons, neutrons, and electrons does chromium have? The “mass number” in “chromium-63” refers to
Hence, chromium has a total of 24 protons. The atomic mass of chromium is 51.9961, so we’ll take the roundup value as 52. And the atomic number of chromium is 24. Subtract the atomic number (24) from the atomic
Study with Quizlet and memorize flashcards containing terms like The atomic number of polonium is 84.The atomic number lead is 82.Which must occur for polonium to be transformed into
112 ZeilenProtons, neutrons and electrons of all elements are mentioned in the table below (You will get the List + Shell diagram of all the elements.)
To determine how many neutrons are in a chromium (Cr) atom, you need to understand the difference between atomic number and mass number. Chromium has an
- Susan Sontag. 100 Seiten | Susan Sontag Lebenslauf
- Frauen In Saudi-Arabien Dürfen Jetzt Alleine Reisen
- Aribert Reimann Gestorben _ Aribert Reimann Lear
- Burping Spiritual Meaning: Uncovering Hidden Messages
- The Best Hiit Workouts To Improve Runner Fitness
- Destiny 2 The Militia’s Birthright
- Diabetes Gestacional Autorreferido
- Regulierungsbehörde Verbietet 0190-Sparvorwahlen
- Effects Not Loading/Working
- Buchungen Rechnungswesen _ Buchungssätze Tabelle
- Celine Bethmann Heidi Klums _ Heidi Klum Neuigkeiten