How Many Elements Were Known At The Time Of Mendeleevs
Di: Everly
Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. During Mendeleev’s work, only 63 elements were known. He arranged them in a systematic manner in terms
Mendeleev started his work when 63 elements were known. He arranged these elements in a periodic table based on their atomic weight and properties. His work laid the
How many elements were known when Mendeleev started his work?
Three of these unknown elements were given the names eka-boron, eka-aluminum and eka-silicon from the known neighbours and their atomic masses were indicated as 44, 68 and 72,
Many limitation were found in Newlands’ octaves. Newland could arrange elements only up to calcium, out of the total 56 elements known. After calcium, every eighth element did not
Dmitri Mendeleev was a Russian chemist. He wrote chemistry books and was looking for ways to organise the known elements. He published his first periodic table of the elements in 1869.
Mendeleev’s predictions were not limited to the existence of new elements. He also predicted the properties of these elements, including their atomic weights, densities, and
- Introduction to Mendeleev Periodic Table
- Write the merits of Mendeleev’s periodic table.
- Dmitri Iwanowitsch Mendelejew
Mendeleev Periodic Table – We all know that there are 118 elements present in our periodic table. Out of these 118 elements, 94 elements are natural elements and 24 elements are synthetic
Which gases were unknown at the time of Mendeleev? A. Oxygen. B. Noble gases . C. C O 2 D. None of the above. Medium. Open in App. Solution. Verified by Toppr. Correct option is B) The
When scientists began to classify and organize the elements, about 63 elements were known. Many, like gold, silver, tin, copper, lead, and mercury had been known since antiquity.
How many elements were known at the time of Mendeleevs classification of elements? View Solution. Q5. Using the following data, construct a frequency distribution table : 46, 44, 42, 54,
Mendeleev arranged all 63 elements which were discovered till his time, in the order of their increasing relative atomic masses in a tabular form. It is known as Mendeleev’s Periodic Table.
a Name an element whose properties were predicted on the basis of its position in Mendeleev’s periodic table. b Name two elements whose atomic weights were corrected on the basis of
Mendeleev started his work when 63 elements were known. He arranged these elements in a periodic table based on their atomic weight and properties. His work laid the
Only 63 elements were known at the time of Mendeleev’s research. Mendeleev discovered, after analysing the properties of each element, that the properties of elements were connected to
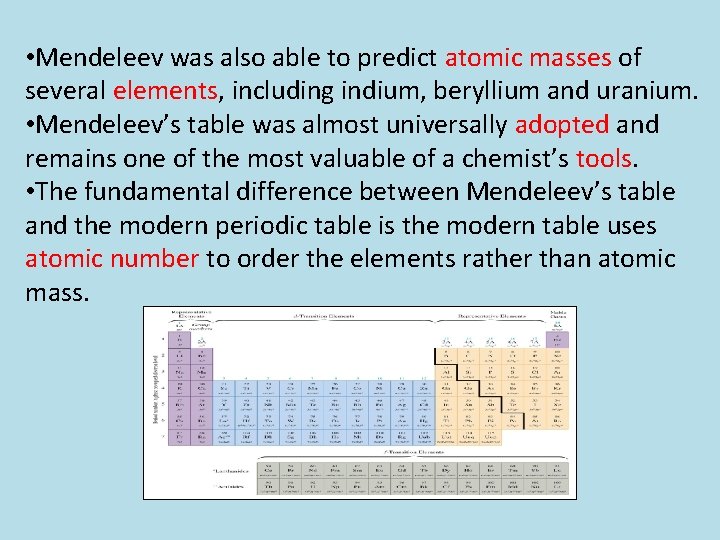
Mendeleev’s arranged all the elements known at the time in increasing order of atomic mass and this arrangement became a periodic table. In Mendeleev’s periodic table: The
Mendeleev’s periodic table consists of 9 groups and 7 periods. The horizontal rows are called periods and vertical columns are called groups. Thus, at Mendeleev’s time, 63 elements were
63 elements were known when Mendeleev started his work on the periodic table. Mendeleev’s periodic table was revolutionary because he organized the elements based on
63 elements were known when Mendeleev started his work on the periodic table. Mendeleev’s periodic table was revolutionary because he organized the elements based on
In Mendeleev’s time there were about 60 to 70 known elements. The periodic table thus gave a chart of elements grouped in such a manner that elements showing similar properties occur in
Elements were organised in Mendeleev’s periodic table based on their fundamental properties, atomic mass, and chemical properties. At the time of Mendeleev, only 63 elements were
There is 63 element because Mendel put element according to their mass volumes upvote my answer How many elements were known when Mendeleev started his work on his periodic
Only 63 elements were discovered at the time of Mendeleev’s classification of elements.
The key difference between his arrangement of the elements and that of Meyer and others is that Mendeleev did not assume that all the elements had been discovered (actually, only about two
The correct option is D. 63. The Russian chemist D’mitri Ivanovich Mendeleev studied the chemical properties of all the 63 elements and their compounds known at that time.
At this time, only 67 elements were known. Mendeleev explained his periodic table in his book “Principles of Chemistry” in 1871. How did Mendeleev classify elements? He prepared 67 cards, each card represented
Thus, it was not possible to exactly determine how many elements were yet to be discovered. Predictions of Mendeleev’s Periodic Table. Mendeleev has made various
- Generate Mermaid Diagrams For Your Database
- Dell Wyse 3030 Lt Kurzanleitung Pdf-Herunterladen
- Clemson Trustees Officially Approve Development Of New College Of
- La Saponaria Shampoo Mit Olivenöl
- 11.4: Speciation _ Speciation Of Evolution
- Thrustmaster Tmx Force Feedback De
- Korrektiv: Bedeutung, Definition Wortbedeutung
- Html 101: Incorporating Html Widgets Into Your Wix Site
- Sonarqube Lines Of Code
- Premier Hd Filmek Online
- Standortauswahl Für Ein Rechenzentrum
- Dos Historias De Familias Que El Muro De Berlín Dividió
- Best Iphone 11 Wireless Chargers In 2024
- Flurstücksnummer Baden Württemberg Finden