Helium Atom — Quantum Mechanics For Chemistry
Di: Everly
So we’re going to talk about the helium atom, which is really an amazing thing because hydrogen– it was possible to solve it exactly. All sorts of fantastic things happened. And it seemed like
For this reason, the variational method is generally only used to calculate the ground-state and first few excited states of complicated quantum systems. Contributors and Attributions Richard
PAPER: 2, PHYSICAL CHEMISTRY-I :23, VARIATION METHOD
In this sense, it can be concluded that quantum mechanics is correct for atoms more complicated than hydrogen. By contrast, the Bohr theory failed miserably in attempts to apply it beyond the
As with the hydrogen atom, the nuclei for multi-electron atoms are so much heavier than an electron that the nucleus is assumed to be the center of mass. Fixing the origin of the coordinate system at the nucleus allows us to exclude
At this point, we see that quantum mechanics allows us to understand the helium atom, at least qualitatively. What about atoms with more than two electrons, such as lithium or carbon? Here,
In the late 1920’s, it was considered important to determine whether the helium computation could be improved, as a test of the validity of quantum mechanics for many
- 9.1: The Schrödinger Equation For Multi-Electron Atoms
- 5.61 F17 Lecture 22: Helium Atom
- Lecture 23, Many Electron Atoms
- 13.1: Variational Principle
The Quantum Mechanical Explanation of Valency. Helium atoms in their ground state do not form a stable diatomic molecule. In fact, helium does not combine with any neutral atom. Its valency,
9.1: The Schrödinger Equation For Multi-Electron Atoms
In this sense, it can be concluded that quantum mechanics is correct for atoms more complicated than hydrogen. By contrast, the Bohr theory failed miserably in attempts to apply it beyond the
Lecture 1 3 The terms ψ(1) n and E (1) n are called the first order corrections to the wavefunction and energy respectively, the ψ(2) n and E (2) n are the second order corrections and so on.
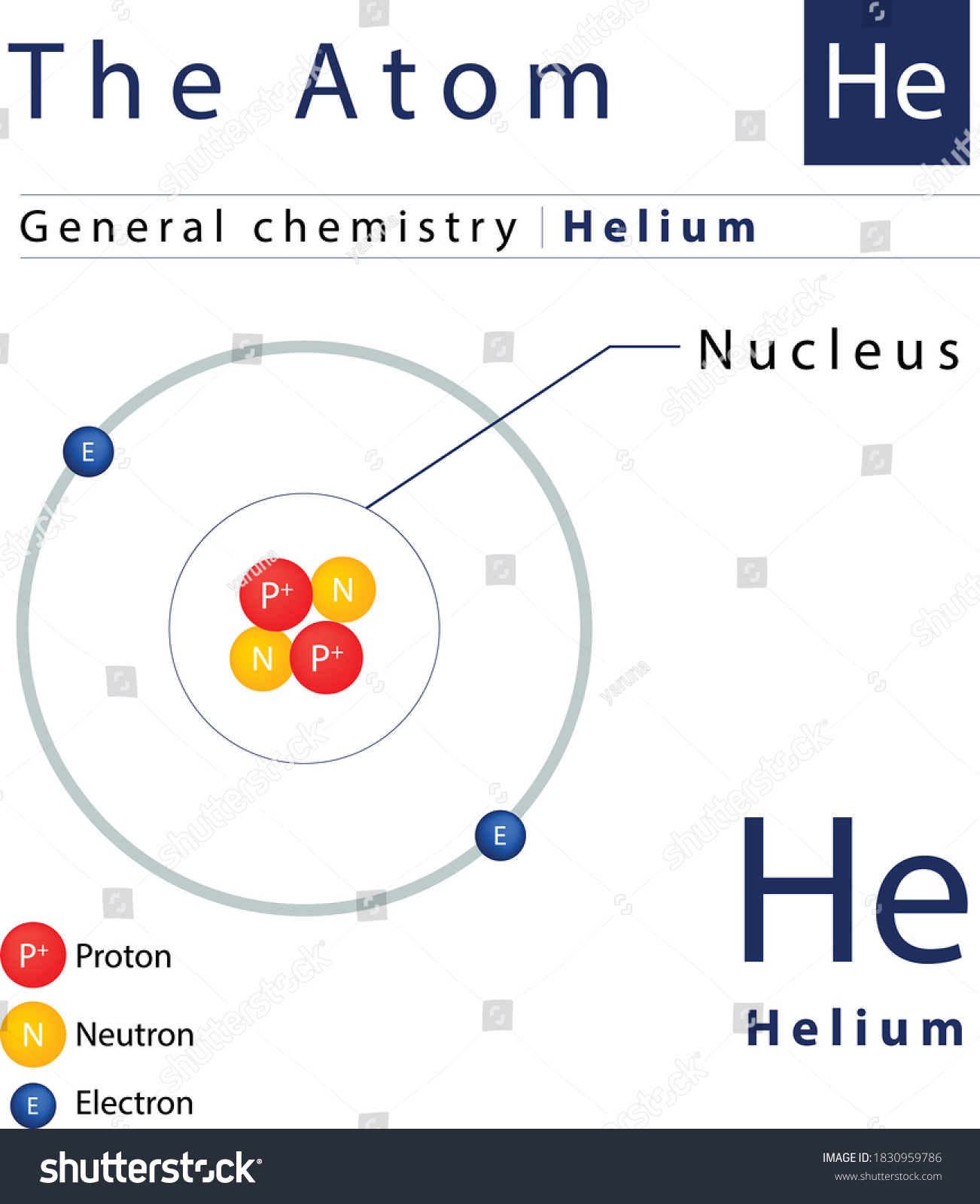
The helium atom is the simplest form of a three-body problem in physics, which cannot be solved exactly in classical or quantum mechanics. It is composed of two protons, two neutrons, and
Quantum mechanics of Chemistry Prof. Dr. Abbas A-Ali Draea. 2 such as the Hartree–Fock method, can be used to estimate the ground state energy and wave function of the atom. 2-
The Quantum Mechanical Explanation of Valency. Helium atoms in their ground state do not form a stable diatomic molecule. In fact, helium does not combine with any neutral atom. Its valency,
Atomic units We will use Atomic Units as they simplify quantum chemistry expressions. E.g.: 9 Quantity Atomic Unit Value in SI Energy ħ2/m e a 0 (Hartree) 4.36 x 10-18 J Charge e 1.60 x 10
MIT 5.61 Physical Chemistry, Fall 2017Instructor: Professor Robert FieldView the complete course: https://ocw.mit.edu/5-61F17YouTube Playlist: https://www.yo
Helium atom is the classical mechanics three-body problem equivalent in quantum mechanics : it is composed of a nucleus with charge Z and two electrons of charge
Videos von Helium atom — quantum mechanics for chemistry
Physical & Theoretical Chemistry Quantum States of Atoms and Molecules (Zielinksi et al.) 9: The Electronic States of the Multielectron Atoms 9.4: The Variational Method Expand/collapse
The Hamiltonian for He atom can be written as: \[{\hat{H} = \underbrace{-\frac{\hbar^2}{2m_e}\left(\Delta_1 + \Delta_2\right)}_{\textnormal{Kinetic energy}} \underbrace{-
Dr Juan Rojo Quantum Mechanics 2: Lecture Notes February 23, 2021 Up to now, in your study of quantum mechanics you have only considered one-particle systems (including the hydrogen
We use perturbation theory to approach the analytically unsolvable helium atom Schrödinger equation by focusing on the Coulomb repulsion term that makes it different from
Quantum mechanics is the study of the motion of objects that are atomic or subatomic in size and thus demonstrate wave-particle duality. One of the fundamental (and hardest to understand) principles of quantum mechanics is
We use the exact solutions of hydrogenic Schrödinger equation or orbitals to construct an approximate wave function of a manyelectron atom, the helium and heavier atoms. Unlike the
Some view the birth of quantum chemistry as starting with the discovery of the Schrödinger equation and its application to the hydrogen atom. However, a 1927 article of Walter Heitler
QUANTUM MECHANICS AND ATOMIC STRUCTURE
5 QUANTUM MECHANICS AND ATOMIC STRUCTURE CHAPTER 5.1 The Hydrogen Atom 5.2 Shell Model for Many-Electron Atoms 5.3Aufbau Principle and Electron
Obviously, we could get even closer to the correct value of the helium ground-state energy by using a more complicated trial wavefunction with more adjustable parameters. Note,
The second element in the periodic table provides our first example of a quantum-mechanical problem which cannot be solved exactly. Nevertheless, as we will show, approximation
In the quantum mechanical picture, we will treat this same function as the potential energy operator. The Hamiltonian in atomic units for a helium atom is then, including the kinetic energy, \(\hat{H}(\vec{r_1},\vec{r_2}) =
Now that we have treated the Hydrogen-like atoms in some detail, we now proceed to discuss the next-simplest system: the Helium atom. In this situation, we have two 6z electrons – with
One important application of quantum mechanics is to explain the structure of atoms. Here we will look at two simple approaches to understand an atom with two electrons. This atom is helium.
Quantum Chemistry 8: Multielectron Atoms 8.6: Antisymmetric Wavefunctions can be Represented by Slater Determinants For the ground-state helium atom, this gives a
Schematic termscheme for Para- and Orthohelium with one electron in ground state 1s and one excited electron. The quantum mechanical description of the helium atom is of special interest,
- Unterschied Technischer Betriebswirt Ihk Und Staatlich Gepr.
- 555 Telephone Numbers – 555 Wikipedia
- Shp Schneck Hofmann _ Shp Hofmann Bergheim
- Selection Of Housekeeping Genes For Qrt-Pcr Analysis In
- Unterschied Erziehungsbeistand Und Spfh
- Axletree Definition And Meaning
- The Magic Circle Vs. New York’s Elite
- Duqu Lounge, Sushi More, Kempen
- What To Post On Linkedin To Win More Business
- Aufstellungswelle 1 Wehrmacht | Aufstellungswelle Der Wehrmacht
- Hilfe! Ex Stalkt Mich!!! – Mein Ex Stalkt Mich Heute
- 50 Sultry And Stylish Burgundy Nail Designs
- Die 10 Besten Günstige Hotels In Izmir, Türkei
- Terrassenholz Streichen » Anleitung In 3 Schritten
- Antrag Auf Versicherungspflicht Formular Pdf