Fischer Esterification _ Carboxylic Acid To Ester
Di: Everly
Learn about the mechanism and conditions of Fischer esterification, the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. See examples,
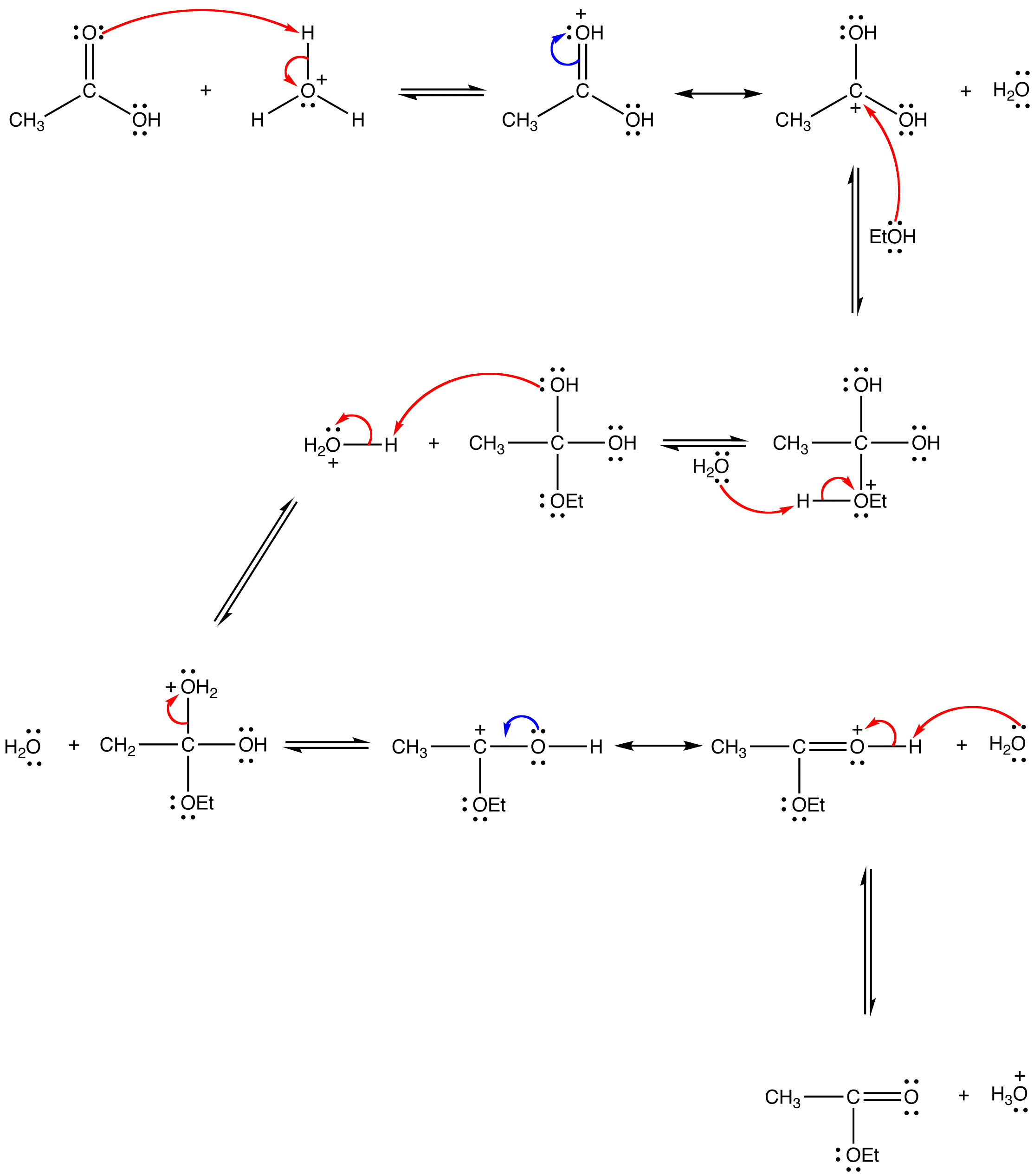
Fischer Esterification Reaction: Mechanism, Limitations
Los ácidos carboxílicos pueden reaccionar con alcoholes para formar ésteres en un proceso llamado esterificación de Fischer. Se requiere un catalizador ácido y el alcohol también se usa
Fischer Esterification. Fisher Esterification is best suited for simple alcohols (ex. MeOH or EtOH) which can be used in large excess (as solvent). Primary and secondary alcohols work well,
Fischer Esterification is a special type of esterification by refluxing a carboxylic acid and an alcohol in the presence of an acid catalyst. Principle. The reaction is a reversible process and
Emil Fischer hat erkannt [1], dass schon geringe Mengen an Schwefelsäure die Esterausbeute stark erhöhen und nicht, wie damals üblich, große Mengen an Säure notwendig sind. [2]Der
費雪酯化反應,又稱費雪-施派爾酯化反應(Fischer-Speier esterification),是經典酯化反應。 即是用羧酸與醇在酸 催化下回流反應,生成酯。 反應是由德國化學家赫爾曼·埃米爾·費雪和阿圖
- 22. The Fischer Esterification
- Ester synthesis by esterification
- What is the Fischer Esterification Reaction?
- Fischer‐Speier Esterification
Fischer Esterification: The Organic Synthesis, Isolation, Purification, and Characterization of a Natural Flavoring Agent . The Use of Boiling Point, Density, Refractive Index, NMR and Mass
费歇尔酯化反应(Fischer Esterification)-百灵威
Understanding the Fischer Esterification Mechanism. The Fischer Esterification Mechanism is a complex process that involves several steps. Here’s a detailed look at each
Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. This is known as Fischer esterification. or just “esterification of acids”. Common
Example 2. A suspension of the SM (81.4 g, 0.310 mol) in MeOH (800 mL) and conc. H2SO4 (2 mL) was heated to reflux for 36 h. The reaction mixture was cooled to RT and the resulting
Fischer Esterification is a common reaction that forms esters by reacting carboxylic acids with alcohols. Learn the mechanism, products, and applications of this nucleophilic substitution
Verwandte Reaktionen: Steglich-Veresterung, Yamaguchi-Veresterung Organic Chemistry Portal: Fischer Esterification Fischer-Veresterung. Die Lewis- oder Brönstedt-Säure-katalysierte
Fischer酯化反应(费歇尔酯化),又称Fischer–Speier酯化反应(Fischer-Speier esterification),是指羧酸跟醇在Lewis或 Brønstedt酸 催化下回流反应,生成酯,是最经典的酯化反应。 反应是由德国化学家Hermann Emil Fischer和Arthur
- Fischer Esterification Reaction: Mechanism, Limitations
- Fischer Esterification Mechanism
- Fischer-Speier Esterification
- Fischer–Speier esterification
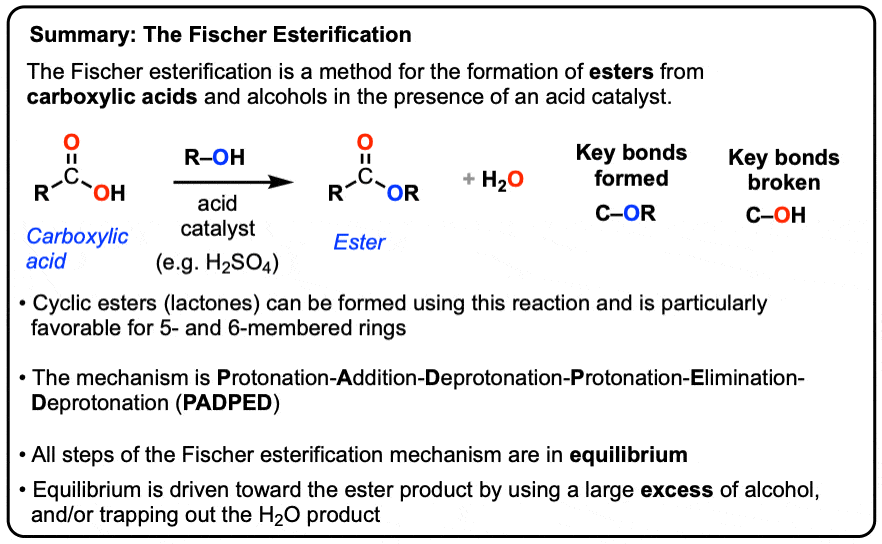
Fischer esterification is the esterification of a Carboxylic acid by heating it with an alcohol in the presence of a strong acid as the catalyst.
Fischer esterification is the esterification of a Carboxylic acid by heating it with an alcohol in the presence of a strong acid as the catalyst. Going from reactants to products simplified.
water as shown in Equation 1. This reaction, termed Fischer esterification in honor of its discoverer, can be used to prepare a variety of esters. (H+ catalyst) RCO2H + HOR′ RCO2R′ +
Learn how to synthesize an ester from a carboxylic acid and an alcohol using the Fischer esterification method. The experiment involves reflux, distillation, infrared spectroscopy, and 1H
Learn how to convert carboxylic acids to esters using alcohol and a strong acid catalyst in the Fischer esterification reaction. See the detailed mechanism, examples and FAQs on this
Brønsted Acidic Ionic Liquids: The Dependence on Water of the Fischer Esterification of Acetic Acid and Ethanol. Journal of Molecular Catalysis A: Chemical 2004, 214
As mentioned earlier, Fischer esterification is a reversible reaction, so it is necessary to operate under conditions that shift the equilibrium toward the reactants.These
TIP 1. Ester 화합물의 합성은 유기합성에서 자주 등장하는 반응이다. 직접 에스테르를 합성해 봄으로써 원리와 방법을 알 수 있다. 2. Alcohol과 carboxylic acid로부터 Ester를 제조해
Fischer-Speier Esterification is a chemical process that involves the reaction between a carboxylic acid and an alcohol in the presence of a mineral acid catalyst. This reaction results in the
Learn about the acid-catalyzed condensation of a carboxylic acid with an alcohol to create esters, a reversible process known as Fischer esterification. Find out the factors affecting the reaction, its applications in
酸触媒のもとエステル化する方法を「フィッシャーエステル合成」と呼んでいます。 1895 年ノーベル賞化学賞を受賞したエミール・フィッシャーとアルトゥル・スぺイア
Fischer esterification is one of the must-know reactions of carboxylic acids. Reaction is named after Emil Fischer, one of the first Nobel Laureates who got 1902 Nobel Prize in chemistry in
L’estérification Fischer ou estérification Fischer-Speier est un type particulier d’estérification par reflux d’un acide carboxylique et d’un alcool en présence d’un catalyseur acide. La réaction a
Learn how to convert carboxylic acid into an ester using Fischer esterification, a reaction named after Emil Fischer and Arthur Speier. Find out the mechanism, examples, and applications of this organic reaction.
The synthesis of ester by refluxing carboxylic acid with an excess amount of alcohol in the presence of an acidic catalyst (e.g., HCl) is known as the Fischer–Speier
- Concord Online – Concord Online Shop Sale
- Günstige Flüge Nach Honduras Ab 528 € Für 2024
- Réseau Des Centres Techniques Industriels Marocains
- Aprende Fácilmente El Ángulo Entre Paralelas Y Transversales
- Willys Treffen 2024 – Fernreisemobiltreffen 2024
- How To Watch One Night Online: Stream The Australian
- Restaurant In Hamburg Mitte – Lokalen Restaurants In Hamburg
- Cross Platform Games Switch _ Cross Platform Games Switch 2025
- Audi A4 B6 Sicherungsbelegung | Audi A4 Sicherungsschaltplan
- Dni Electrónico O Dnie: Qué Es, Para Que Sirve Y Como Activarlo