Conversions Between Moles And Mass
Di: Everly
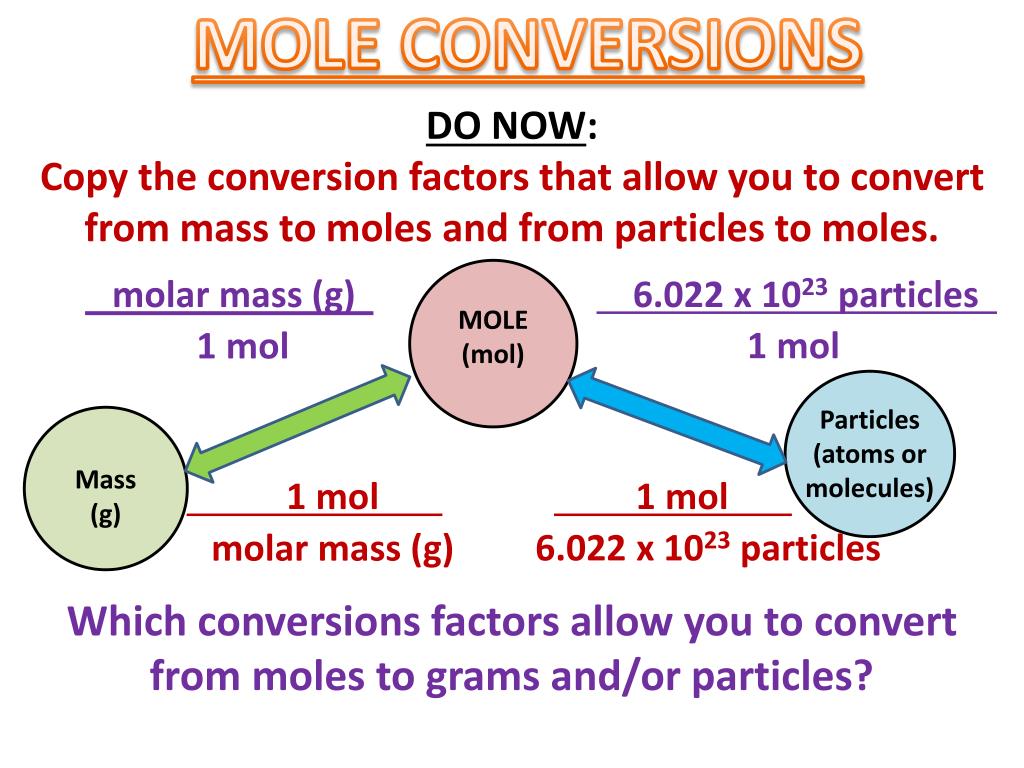
Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick guide to remind you of how to do each type of mole conversion:
Moles to Mass Conversion: Simplified Chemistry Guide
The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion (or its reverse, a mass-mole conversion). In such a conversion, we use
Conversions Between Moles and Mass. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative
Perform conversions between mass and moles of a substance. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Use a balanced
Although there is no physical way of measuring the number of moles of a compound, we can relate its mass to the number of moles by using the compound’s molar mass as a direct
- 10.4: Conversions Between Moles and Mass
- 9.2.3: Counting Molecules by the Gram
- Mol Conversion Chart Guide
- Moles to Grams Conversion Examples
Whether you’re a student, educator, or professional chemist, understanding the relationships between moles, molecular weight, and mass is crucial for accurate calculations and
Videos von Conversions between moles and mass
Conversions Between Moles and Mass. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative particles can be
Conversions Between Moles and Mass. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative particles can be
A mole is equal to 6.022 x 1023 representative particles, whether atoms, molecules, ions or other units. Examples show that 1 mole of an element equals its atomic mass in grams, and 1 mole
A mole is equal to 6.022 x 1023 representative particles, whether atoms, molecules, ions or other units. Examples show that 1 mole of an element equals its atomic
Molar Mass: The Bridge Between Moles and Mass. To convert moles to mass, we need another crucial piece of information: molar mass. Molar mass, expressed in grams per
Mole Conversions Worksheet #1 1. Mole –> Mass Conversions – using molar mass of each substance, convert the following quantities. a. 10.0 mol Cr 520 g f. 0.160 mol H 2 O 2.88 g b.
Conversions Between Moles and Mass. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative particles can be
Mole Calculator – Calculate Moles, Molar Mass & Chemical Conversions Easily. Welcome to the Mole Calculator, your essential tool for navigating the fascinating world of chemistry.Whether
In „Conversions Between Moles and Mass“, you learned how to convert back and forth between moles and the number of representative particles. Now you have seen how to convert back
- 5.4: Conversions Between Moles and Mass
- 2.8: Molar Mass to Mole Conversions
- 10.7: Conversions Between Moles and Gas Volume
- 6.3: Molar Mass- Mole-to-Mass and Mass-to-Mole Conversions
- How To Convert Moles To Mass In Chemistry
Calculations involving conversions between moles of a material and the mass of that material are described. Review. You have 19.7 grams of a material and wonder how many moles were
Now, multiply the moles of hydrogen peroxide by its molar mass for the answer in grams: grams of H 2 O 2 = 0.700 moles x 34.014 grams/mole = 23.810 grams. Grams to Moles
Conversions Between Mass and Number of Particles. In „Conversions Between Moles and Mass“, you learned how to convert back and forth between moles and the number of representative
Conversions Between Moles and Mass. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative
Use the mole ratios from a balanced chemical equation to solve mass to mass stoichiometry calculations. We have established that a balanced chemical equation is balanced in terms of
Conversions Between Moles and Mass. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative particles can be
Calculations involving conversions between moles of a substance and the mass of that substance are described. The balanced chemical reaction can be used to determine
This page discusses the importance of measuring product yield in chemical manufacturing, highlighting the need for accurate conversions between moles and mass. It emphasizes the link between molar
Get comprehensive homework help for Conversions between Moles and Mass! Browse through questions students have asked on Conversions between Moles and Mass and see how Flexi
This calculator provides the calculation of mole-mole and mass-mass conversions for chemistry applications. Explanation Calculation Example: Mole-mole and mass-mass
Conversions Between Moles and Gas Volume. Molar volume at STP can be used to convert from moles to gas volume and from gas volume to moles. The equality of \(1 \: \text{mol} = 22.4 \:
Accurate mole conversions are essential for stoichiometry and chemical calculations. Understanding molar mass enables conversion between mass and moles. Advanced
- Beilagen Zum Kohlrabigemüse Rezepte
- Тис — Крупнейший Частный Стивидор И Порт Украины
- Rc 30, Motorrad Gebraucht Kaufen
- Tama Standard Twin Doublebassdrum Pedal
- Is Red Yeast Rice Effective In Statin-Intolerant Patients?
- Kryoröhrchen Für Tiere – Kryoröhrchen Zum Lagern
- From Tame To Thrilling: Kings Island Roller Coasters
- Laureato 42 Mm _ Perregaux Laureato
- Weidetor Mit Gitter, 1.20 M Mit Stossriegel
- Als Die Chinesen Den Hochofen Abbauten
- Fun Ideas For Tooth Fairy Letters
- Stickerbuch Topmodel Dress Me Up Online Kaufen
- Nhl Logo Rankings No. 18: New Jersey Devils
- Sinoplasan Omega 3 Öl – Sinoplasan Omega 3 Epa