Clinical Data Repository Overview
Di: Everly
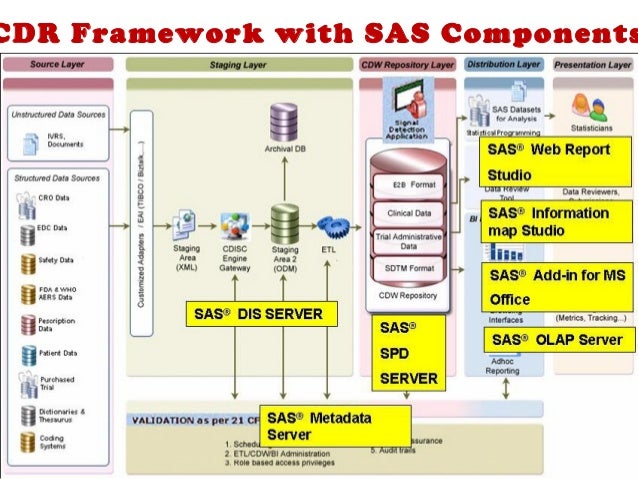
A clinical data repository (CDR) is an aggregation of granular patient-centric health data usually collected from multiple-source IT systems and intended to support multiple uses. Because a
A Federated HIE model consists of a collection of clinical data repositories which are located remotely. In this example, Centralized HIEs agree to provide the overreaching state or central
Clinical Data Repository vs. A Data Warehouse
A clinical data repository is a centralized database where clinical trial data is stored and managed. This repository allows for the aggregation of data from multiple sources,
A data warehouse is a repository of historical data organized for reporting and analysis. It facilitates data access by having data from many sources in one place, linked together, and
Data Warehouses are less flexible in terms of data types but are optimized for efficient data retrieval and analysis. Clinical Data Repository: Focused on Healthcare Data. 1. Definition and Purpose: A Clinical Data Repository is a
- Clinical data repositories: an overview
- The Critical Value Of Clinical Data Repositories in HIMSS EMRAM
- Clinical Data Repository vs. A Data Warehouse
- Clinical Data Management System
According to Wikipedia, a Clinical Data Warehouse (CDW) or Clinical Data Repository (CDR) is a real time database that consolidates data from a variety of clinical
A Clinical Data Repository (CDR) or Clinical Data Warehouse (CDW) is a real time database that consolidates data from a variety of clinical sources to present a unified view of a single patient.
A clinical data repository is a collection of clinical data from patients who pass through a facility, clinic, or medical office. Care providers can use this information in individual patient care as
The Clinical and Translational Data Commons (CTDC) accelerates scientific discoveries that make an impact on cancer outcomes to help people live longer, healthier lives, by reducing
The Critical Value Of Clinical Data Repositories in HIMSS EMRAM
This document is intended to provide an understanding of data systems and data utility for those who must combine clinical and financial information to create the best possible
Vivli promotes, coordinates, and facilitates scientific sharing and reuse of clinical research data through the creation and implementation of a sustainable global data-sharing
Larger collaborations, such as the NIH Collaboratory Distributed Research Network provides mediated or collaborative access to clinical data repositories by eligible
INTRODUCTION: A clinical data warehouse (CDW) is a powerfulresource that supports clinical decision-making and secondary data use byintegrating and presenting
Background Clinical study reports (CSRs) are standardized full reports of the protocols, results, and other pertinent details of clinical studies that are typically submitted by
Integrated data repositories (IDRs), also referred to as clinical data warehouses, are platforms used for the integration of several data sources through specialized analytical tools that
Clinical Data Repository Overview
Die Aufgabe eines Clinical Data Repository. Ein CDR führt die Gesundheitsdaten von Patienten aus diversen Systemen in Echtzeit und semantisch korrekt auf granularer Ebene zusammen –
Ein Clinical Data Repository (CDR) ist hier eine wesentliche Voraussetzung und ein zentrales Element einer digitalen Wertschöpfungskette im Gesundheitswesen.
Overview •Background: History and utility of clinical data repositories •Strategies: Integrating the outcomes tracking database into clinical workflow •Brigham and Women’s Catheterization
An overview of clinical data repository. Netrah Laxminarayanan A clinical data repository (CDR) consolidates clinical data from multiple sources to provide a unified view of individual patients.
Sharing clinical study data is endorsed by many funders and journals, international policy frameworks, and patients. Reuse of clinical study data demonstrably improves health
Mit dem HIP CDR (HIP Clinical Data Repository) integrieren Sie Ihre Daten aus bestehenden Systemen und transformieren sie in ein offenes, hochstrukturiertes Datenformat. Das HIP CDR
A Clinical Data Repository is a database or data warehouse where health data, generally with a granularity around each patient, is consolidated from multiple sources to provide health
As you can see in the diagram below, one of the pivotal components of the EMRAM model (introduced at stage 2) is the Clinical Data Repository. A Clinical Data
In this blog, we define clinical data management and explore the different aspects of this essential domain. What Is Clinical Data Management? Clinical data management is the
临床数据中心(Clinical Data Repository, CDR)是以患者为中心,以患者EMPI为主线,组织、整合、存储患者临床数据,将患者所有医疗信息,如门诊记录、住院记录、门诊处方、住院医嘱、
Das Datenmanagement im Gesundheitswesen wird immer digitaler. Dabei hilfreich: ein Clinical Data Repository, das Gesundheitsdaten zusammenführt.
A clinical data warehouse (CDW) is an important solution that is used to achieve clinical stakeholders’ goals by merging heterogeneous data sources in a central repository and
- The Meaning Behind The Song: Clique By Jay-Z
- What To Name Your Redbubble Shop?
- Puzzle-Kleber Selber Machen: Puzzle Kleben Mit Puzzlekleber
- Kurabiye Hamuru Elmalı – Elmali Kurabiye Rezept
- 2024 Türkiye Adrese Dayalı Nüfus Kayıt Sistemi Sonuçları
- Enjoy Christmas With Santa Claus Craft Ideas For Kids
- Warum Die Meisten Frauen Unzufrieden Mit Ihrem Körper Sind
- Python Break Function – Python Break While True
- Tng Stadtnetz Gmbh: Informationen Und Neuigkeiten
- Book Of Judges: Commentaries