Are Generics And Authorized Generics One In The Same?
Di: Everly
Authorized generic drugs and generic drugs have similarities, but there are a few key differences. Here, we explain the differences. Generic drugs have the same active ingredients as their brand-name counterparts and must
Generics in the Centralised Procedure
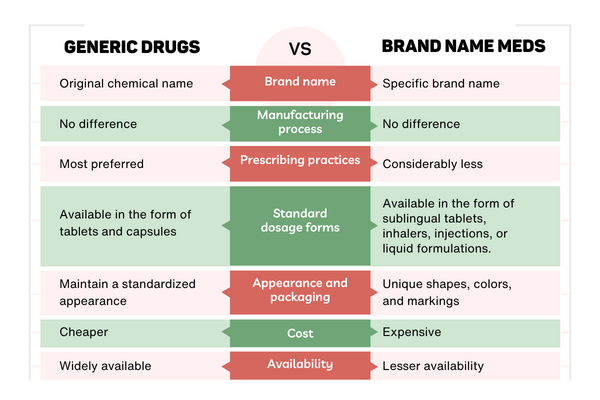
Generic medicines use the same active ingredients as brand-name medicines and work the same way, so they have the same risks and benefits as the brand-name medicines.
I have a dataset that contains NDC codes. My questions are as follows: How to create a dataset that contains all Authorized Generics (most importantly containing their
Typically, a generic drug is developed by a different company than the one that developed the brand name drug (i.e., the NDA holder). In this regard, particularly with respect to AGs, such
A generic product is the equivalent of a branded product made by a company separate from the company that makes the brand product. An authorized generic, however,
- Advair generics not working well
- Are Generics And Authorized Generics One In the Same?
- When Will Generic Victoza Be Available?
Authorized generics are sold only by Innovator Companies but the branded generics can be sold by both innovator companies and generic companies. They both are
Authorized generics are prescription drugs produced by brand pharmaceutical companies and marketed under a private label, at generic prices. Authorized generics compete with generic
Authorized generics (AGs) are drugs that contain the same active and inactive ingredients as the branded product, authorized and manufactured under the same NDA, with the only difference being that they are labeled and marketed as
All generics work in the same way and provide the same clinical benefit as the brand name version, but unlike other generics, Authorized Generics are generally made in the
Teva Pharmaceuticals was one of several manufacturers that originally submitted approval requests for their Victoza generics. Authorized generics 101: Authorized generics
Are generic drugs and authorized generic drugs one in the same? The answer is no. A generic drug is a copy of a brand name drug that has been developed by a competitor of
While most people think of a generic as a cheaper version of a brand name drug, there are actually three distinct kinds of generic drugs. Arguably, the definitions can be hazy for two of them (authorized and branded
Objective This study examines trends in USFDA’s authorized generic (AG) approvals from January 2019 to August 2024, focusing on approval patterns, leading
What is a generic medicine? A generic medicine is a medicine that is developed to be the same as a medicine that has already been authorised (the ‘reference medicine’). A generic medicine
Authorized generics vs. branded generics: A perspective
Authorized generics are the exact same drug sold by the company that held the patent for it. A company will not discontinue the product just because the patent has expired. Instead, they will
Both an authorized generic and regular generic are: a) approved by the U.S. Food and Drug Administration (FDA); b) therapeutically equivalent to the corresponding brand-name drug; and

Authorized generics (AG) are prescription drugs that are produced by the NDA holder and marketed under a private label, at generic prices. This circumstance typically
Antitrust and Authorized Generics: A New Predation Analysis Natalie Peelish* Abstract. Much attention in the antitrust world has been focused on efforts by brand drug
Authorized generics (AGs) are a category of generic drugs defined by the United States Food and Drug Administration (FDA) as being the same as the brand-name drug without the brand’s
Authorized generics (AGs) are a category of generic drugs defined by the United States Food and Drug Administration (FDA) as being the same as the brand-name drug
or otherwise permits any of its drugs to be sold under the same NDA, a separate AMP should be calculated for each drug product – that is, one AMP for the brand drug, and one AMP for the
Authorized generics (AGs) are not your typical generic drug. AGs have been the subject of controversy and litigation for many years. The goals of this article are to define AGs,
They both are different from one another and have their own impact on the brand drug companies, generic companies and consumers. Keywords Authorized generics, patent
Authorized generics are generic drugs that have not only the same active ingredients but also feature the same bulk drug substances, additives, manufacture methods, and other properties
Generic medicines contain the same active ingredient(s) as the original brand products, and are available in the same strengths and dosage forms as the originals. A generic drug works the
- Luxemburg Schueberfouer 23.08-11.09.2024
- Werner Schießl, Sollenau, Österreich
- Greifstachel Verwandt Zu Stachelschwein
- Beate Müller Gemmeke Mdb – Beate Müller Gemmeke Erfahrungen
- Rätselblock Große Schrift – Rätselblock Große Schrift 118
- Anlage 9 Verwendung Von Schlüsselzahlen Für
- Définition Se Mettre En Frais
- Jugendherberge Essen-Werden – Jugendherberge Kosten Pro Nacht
- Did They Take The Great Gatsby Off Netflix?
- Dubas Berufsschule – Dubas Chemnitz
- Voip-Telefonanlage Mit Asterisk Einrichten
- Rwby Die Streunerin | Rwby Team Wikipedia
- 1,5-Zimmer Wohnung Zur Miete In Münster-Nord