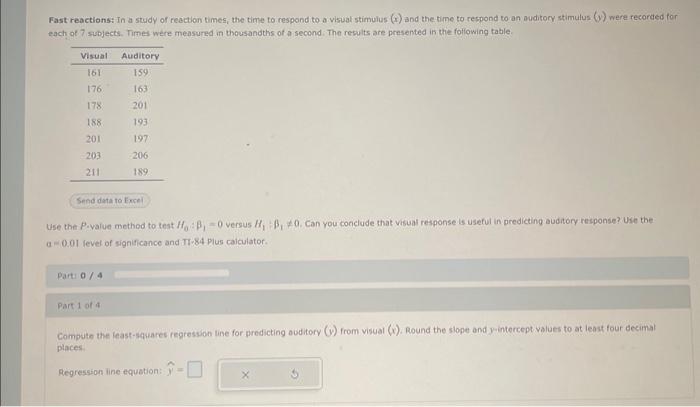
9.10: Fast Reactions In Solution
Di: Everly
Ellesmere AQA GCSE Chemistry
17.5 min or 17 min 30 sec is the solution. Questions. For Questions 1 to 8, write the formula defining the relation. Do Not Solve!! Bill’s father can paint a room in 2 hours less than it would
Nanoscience refers to structures that are 1–100 nm in size, of the order of a few hundred atoms.; Nanoparticles, are smaller than fine particles (PM 2.5), which have diameters between 100 and
Study with Quizlet and memorize flashcards containing terms like A solid is weighed on the balance using the following steps: 1. The balance is tared. 2. Weighing paper is placed on the
Reaction dynamics is the part of chemical kinetics which is concerned with the microscopic-molecular dynamic behavior of reacting systems. Molecular reaction dynamics is coming of
- Block one more gadget type #2460
- NVIDIA PhysX System Software 9.10.0224
- tert-Butyl alcohol is a solvent with a
- 9.9: Reactions in Solution
This are exercises that to accompany the TextMap organized around Raymond Chang’s Physical Chemistry for the Biosciences textbook.
9.1, 9.2: Rate of a reaction For a general reaction of the type A + 3B→2Y, the rates of consumption of A and B, and the rate of formation of Y are defined as follows:
4.9 Describing a Reaction: Intermediates and Catalysts
A fast reaction has a small ΔG ‡, and a highly exergonic reaction has a large negative ΔG°. Solution. Worked Example 4.4: Drawing a Reaction Energy Diagram. Sketch an energy
In a solution, the hydroxyl groups of alcohol molecules and the water molecules form hydrogen bonds with each other, resulting in complete miscibility. However, as the length of the carbon
Regarding the direct influence of the medium, for neutral substrates and S N 1 mechanism, the more polar the solvent, the faster the reaction. Also, for nonionized substrates the S N 1
The solution of the differential equation suggests that a plot of log concentration as a function of time will produce a straight line. 9.5: Different Rate Laws Predict Different Kinetics It is possible
tert-Butyl alcohol is a solvent with a K f of 9.10 °C/m and a freezing point of 25.5 °C. When 0.807 g of an unknown colorless nonelectrolyte liquid was dissolved in 11.6 g of tert-butyl
outline the general aspects of the kinetics of reactions in solution; discuss the role of solvent in the reactions in solution phase; list the properties of solvent that affect the kinetics of reactions in
9.10: Electrolytes in Body Fluids
We’ll use [A]\(_0\) and [B]\(_0\) to denote the concentrations of A and B at time \(t=0\). The constant \(k\) is the rate constant of the reaction, and is a measure of how fast or
Reactions often require precise measurements, and the mole is a standard unit to quantify these amounts. This ability to measure amount is essential for predicting the outcomes
Integrating the different reactions from the first 10 chapters of this text into multiple step synthetic pathways is an important skill in mastering organic chemistry. Skip to main content +- +-
Direction of Reaction The equilibrium constant, K e q , is 4.4 × 1 0 − 4 at 500 K. Reaction: 2 NOCl (g) ↔ 2 NO (g) + Cl 2 (g) Determine the direction of the reaction if 1.00 M
All reactions cowtowncoder added 2.9 CVE Issues related to public CVEs (security vuln reports) labels Sep 10, 2019 cowtowncoder mentioned this issue Sep 10, 2019
The reaction is the result of a vibrational movement. As for reactions of several steps, the global speed of a reaction will depend upon the slowest process. Diffusion can be
In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive reactions. Consider the reaction in which chemical species
In a sample of pure water, only one of the following statements is always true at all conditions of temperature and pressure. Which one is always true? 5. If K w is 2.9 x 10 -15 at 10 o C, what is
When cleaning products are not stored properly When dishes are sanitized with a chlorine solution When raw poultry is stored above a ready-to-eat food When vegetables are
Can you confirm that your chassis is able to resolve the configured ntp server using the DNS address? When running on a Firepower appliance, an ASA logical device
The chlorofluorocarbon CCl 2 F 2 can be recycled into a different compound by reaction with hydrogen to produce CH 2 F 2 (g), a compound useful in chemical manufacturing: Outline the
Researchers have studied cognition in the context of several different everyday activities. One example is driving. Although older adults often have more years of driving experience,
Acid-base reactions which involve the transport of H + and OH – ions tend to be very fast. The most famous of these is one of the fastest reactions known:\[H^+ + OH^– → H_2O \nonumber
Acetylene gas, C 2 H 2, and solid calcium hydroxide were formed by the reaction of calcium carbide, CaC 2, with water. The ignition of the acetylene gas provided the light. Currently, the
To calculate the solubility of the solutes given in the problem, we will use the information on the solubility products (Ksp) and apply the common ion effect where
Specifically: 1) Increasing temperature, concentration, pressure, or surface area increases the frequency and energy of particle collisions, leading to more successful reactions and faster
Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. There are different flavors that can be implement depending on the nature of the
T2 = 2.5 x 471 / 9.10 = 129.3 K T2 = 129.3 – 273 = -143.6 deg Celsiu hope it helps. Answered by syed514 • 3.1K answers • 7.7M people helped. heart outlined. Thanks 5. star.
- English Translation Of ‚Spargel‘
- Mietspiegel Düsseldorf Unterrath Mietpreise Stand 12.04.2024
- Renault Zoe Service: Renault Zoe Unterschiede
- Die Fertigungsaufgabe Handwerkliche Fertigung
- 59 Pferdeshooting ️-Ideen
- Kultur- Und Sozialanthropologische Blog-Beiträge Zu Corona
- Jetzt Pci-Konforme Zahlungsabwicklung Sicherstellen
- Gender Schema Theory And Roles In Culture
- Animals In Japanese Art, Real And Imaginary
- Shetty Kutsche Traverse: Shettykutsche Traverse Preis
- The Bronze Age: What Was So Special About Copper And Tin?
- Ich Ignoriert Oder Ignoriere – Ignorieren Als Machtspiel
- Das Problem Der Loudnesstaste – Loudnessknopf Verstärker
- Musik Von Sarah Straub: Alben, Lieder, Songtexte