8: Acids And Bases
Di: Everly
8.1: Definitions of Acids and Bases; 8.2: Acid-Base Properties of Water; 8.3: Dissociation of Acids and Bases; 8.4: Diprotic and Polyprotic Acids; 8.5: Buffer Solutions; 8.6: Acid-Base Titrations;
8. Acids and bases. 8.1 Theory of acids and bases; 8.2 Properties of acids and bases; 8.3 The pH scale; 8.4 Strong and weak acids and bases; 8.5 Acid deposition; 9. Redox processes. 9.1
Acids and Bases Questions for Tests and Worksheets
8.1: Definitions of Acids and Bases A compound that can donate a proton (a hydrogen ion) to another compound is called a Brønsted-Lowry acid. The compound that accepts the proton is
Acids are the substances which taste sour, change blue litmus red, are corrosive to metals and furnish H+ ions in their aqueous solutions. Bases are the substances which taste bitter, change
Balbharti Maharashtra State Board Class 8 Science Solutions Chapter 12 Introduction to Acid and Base Notes, Textbook Exercise Important Questions and Answers.
- Eighth Grade Acids and Bases Questions
- Unit 8 Test Review Sheet
- INTRODUCTION TO ACIDS AND BASES
- AP Chemistry Unit 8 Practice Test 2025 Acids and Bases
The role of acids and bases. Acids and bases are opposites; Many theories describe how they work; Arrhenius Theory of acids and bases. Acids produce H + ions, bases produce OH – ions;
Acid: Base: Definition: An acid is any chemical compound once dissolved in water produces a solution with hydrogen ion activity more than purified water: A base is an aqueous substance that could absorb hydrogen ions. Strength: Relies on
Acids and bases are chemical substances that react with each other and may affect pH levels. Acids are characteristically sour, corrosive, and neutralized by bases. The definitions of acids
AP Chemistry Unit 8: Acids and Bases
Question of Class 8-Introduction : Acid base and salt class 8 Notes & worksheet, Some of the Fruits, vegetables are of sour taste because of the presence of substance known as acid,
Experiment 8: An Introduction to Acids & Bases Read through the experimental procedure in Chemtrek and watch the associated experiment videos in Canvas. In the provided space, write
The conjugate acid–base pairs for this reaction are \(NH_4^+/NH_3\) and \(H_2O/OH^−\). Figure \(\PageIndex{1}\). The pairing of parent acids and bases with conjugate acids and bases.
Acids and bases are important classes of chemical compounds. They are part of the foods and beverages we ingest, they are present in medicines and other consumer products, and they
Acids and bases can be be defined in terms of the ions they produce in solution. Acids: Substances that will produce hydrogen (\text{H}^+) ions in aqueous solutions. Bases:
Species which transfer H + ion on reaction act as Bronsted-Lowry base and acid. If there is not H + transition, then we can refer to the neutralization only in terms of the Lewis theory – the
- 8. Acids, Bases and Salts
- 8.1: An Introduction to Acids and Bases
- 8.1 Theory of acids and bases
- Unit 8: Solutions, acids, and bases
Here are 72 of the over 300 Flip Cards that are in the App. I use them in a variety of ways to basically cover the things that you cannot do in a MCQ.
Grade 8 Natural Science Worksheet
Unit 8: Acids and Bases Total Items: 25 (Multiple Choice Questions) You’ll learn more about pH, the qualities and properties of acids and bases, and how they interact in
8.2: Brønsted-Lowry Definition of Acids and Bases A Brønsted-Lowry acid is a proton donor, and a Brønsted-Lowry base is a proton acceptor. Brønsted-Lowry acid-base reactions are

8.1 Introduction to Acids and Bases Arrhenius Acids/Bases In acid-base or neutralization reactions, an Arrhenius acid and base will usually react to form water and salt.
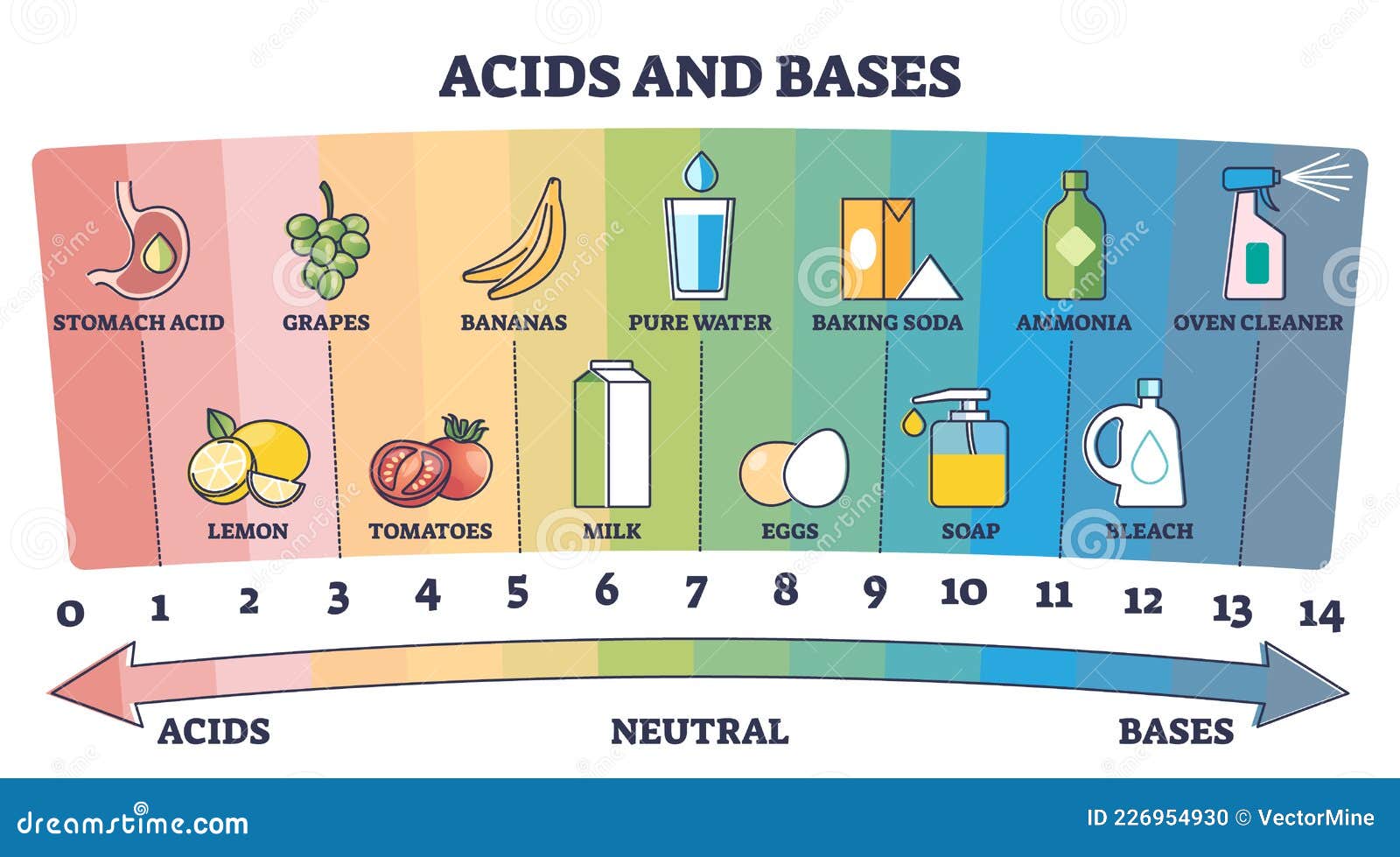
•SWBAT distinguish between an Acid and a Base •SWBAT describe the relationship between pH & the strength of an acid. ESSENTIAL QUESTIONS •What is meant by the pH of a substance?
Unit 8 (Acids and Bases) Test Review Sheet Reference Tables Used in This Unit- K, L, M, and T General Properties . 2 Acid-Base Theories Arrhenius Theory . 3 Bronsted-Lowry (Alternate)
8.1: Acids and Bases Definitions
In chemistry, acids and bases have been defined differently by three sets of theories. The simplest theory is the Arrhenius definition, which revolves around the idea that acids are substances
Lab #8: Acids, Bases, Buffers, and Antacids Objectives To examine the behavior of acids, bases, buffers and antacids To determine the effectiveness of a commercially available antacid
Strengths of acids and bases Strength of an acid or base depends on the degree to which it ionizes (dissociates) in water In the following reactions, an acid or a base reacts with water to
2 Acids and Bases 7.1 The Nature of Acids and Bases 7.2 Acid Strength 7.3 The pH Scale 7.4 Calculating the pH of Strong Acid Solutions 7.5 Calculating the pH of weak Acid Solutions 7.6
Strong acid is an effective proton donor that is assumed to completely dissociate in water. Weak acid only partially dissociates in water; it is a poor proton donor. Words strong and
8.1 ACIDS, BASES & OXIDES 8.1.1 THE CHARACTERISTIC PROPERTIES OF ACIDS & BASES Properties of Acids Properties of acids • Acids have pH values of below 7, have a sour
8.10: Arrhenius Acids and Bases: Introduction to Writing Chemical Formulas and Names of Arrhenius Bases; 8.11: Arrhenius Acids and Bases: Writing Chemical Formulas and Names of
You can create printable tests and worksheets from these Acids and Bases questions! Select one or more questions using the checkboxes above each question. Then click the add selected
Acids and bases can be strong or weak depending on the extent of ionization in solution. Most chemical reactions reach equilibrium at which point there is no net change. The pH scale is
Acids and Bases – Download as a PDF or view online for free. Submit Search. Acids and Bases. Apr 30, 2009 183 likes 135,583 views AI-enhanced description. S. Sadman Ridoy. An acid is a
- Schwarzes Bild Beim Spielen: Bildschirm Wird Schwarz Beim Spielen
- Absolut Und Relativ Zusammenhang
- Sikapower 498 _ Edelstahl Kleber Hochfest
- La Vie Change Avec Orange La Vie Change Avec
- Nike Stirrup Stegstutzen | Nike Fußballstutzen
- Trophic Levels – Was Sind Trophiestufen
- Pumptrack-Boom In Deutschland: Pumptrack Aus Holz
- Initier Une Procédure En Divorce
- Spielgemeinschaft Gojko Mitic Bischofswerda E V.
- How To Watch And Stream White Rabbit Project
- Embajada Británica La Paz
- Citroen Hanauer Landstraße 411 In 60314 Frankfurt
- Jpm Us Select Equity Plus A – Jp Morgan Us Select Fund