4.3.2. Hydrogen Bond Analysis
Di: Everly
QTAIM analysis gave the binding energy of the HBs for the systems in the range of −2.1 to −3.6 kcal/mol, which is classified as weak hydrogen bonds. Aromatic thiols are
Classes¶ class pmda.hbond_analysis.HydrogenBondAnalysis (universe, donors_sel = None, hydrogens_sel = None, acceptors_sel = None, d_h_cutoff = 1.2, d_a_cutoff = 3.0,
Calculating hydrogen bonds: the basics

Similarly, poly(2,2,3,3,3-pentafluoropropyl methacrylate) is immiscible with PVPh, while their blends change from heterogeneous to homogeneous on incorporation of a sufficient
B.16 3-CENTER, 4-ELECTRON HYPERBOND SEARCH B-166 B.16.1 Introduction B-166 B.16.2 Sample Output B-168 B.17 NBCP: NATURAL BOND CRITICAL POINT ANALYSIS B-170
Perform an analysis of hydrogen bonds in a Universe. Set up atom selections and geometric criteria for finding hydrogen bonds in a Universe. Hydrogen bond selections with donors_sel,
- Quantitative Analysis of Multiplex H-Bonds
- Calculating hydrogen bonds: advanced selections
- PyMOL: Determination of hydrogen bonding interactions
Step 6: Detailed Hydrogen Bond Information. Another valuable file generated from this analysis is the hbonds-details.dat file, containing specific details about the identified
def guess_donors (self, select = ‚all‘, max_charge =-0.5): „““Guesses which atoms could be considered donors in the analysis. Only use if the universe topology does not contain bonding
In the current study, we provide a comprehensive quantitative analysis of serine and threonine side chains H-bonding to backbone carbonyls in over-coordinated and bifurcated H-bonds. Our
Parameters—–current : list The current water bridge being analysed is a list of hydrogen bonds from selection 1 to selection 2. output : dict A dictionary which is modified in-place where the
For example, to find hydrogen bonds involving a protein and any water molecules within 10 Å of the protein (which may be useful for subsequently finding the lifetime of protein-water hydrogen
AnalysisBase): „““ Perform an analysis of hydrogen bonds in a Universe. „““ def __init__ (self, universe, donors_sel = None, hydrogens_sel = None, acceptors_sel = None, d_h_cutoff = 1.2,
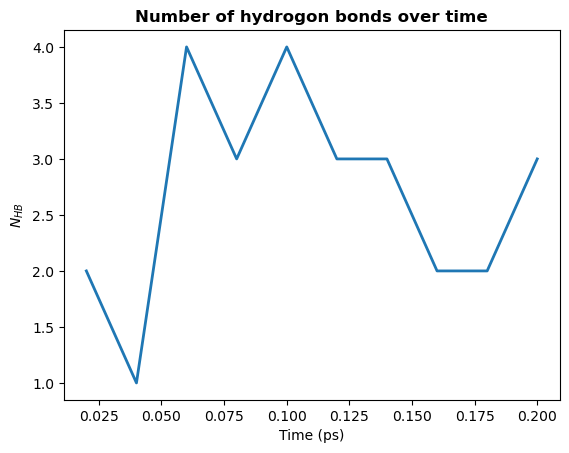
Hydrogen bond analysis is used to investigate the stabilization mechanism of water molecules. The number of hydrogen bonds after 100 % ethanol removal was analysed
To analyze the hydrogen bond structure of O c atoms, we focus on O c atoms which are located within the first hydration shell of O s atoms (4.3 Å away from the O s atoms, Fig. 4).
AnalysisBase): „““ Perform an analysis of hydrogen bonds in a Universe. „““ def __init__ (self, universe, donors_sel = None, hydrogens_sel = None, acceptors_sel = None, d_h_cutoff = 1.2,
Calculates the time autocorrelation function, Cx(t) C x (t), for the hydrogen bonds in the selections passed to it. The population of hydrogen bonds at a given startpoint, t0 t 0, is evaluated based
Hydrogen atoms bonded to a donor are searched with one of two algorithms, selected with the detect_hydrogens keyword. Searches for all hydrogens (name “H*” or name “ [123]H” or type
The intermolecular electron transfer occurs from lone pair LP(F) orbitals to the antibonding σ*(C53-H54 and C16-H17) orbitals with the corresponding E(2) values 3.27 and
本单元提供了在宇宙中寻找和分析氢键的方法。 这个 HydrogenBondAnalysis 类是原始版本的新版本 MDAnalysis.analysis.hbonds.HydrogenBondAnalysis 从模块中初始化
For example, to find all hydrogen bonds in water:: import MDAnalysis from pmda.hbond_analysis import HydrogenBondAnalysis as HBA u = MDAnalysis.Universe(psf, trajectory) hbonds =
From the perspective of utilization, hydrogen can work as a direct energy or the fuel in fuel cells to drive various power systems in various vehicles [25, 26], ships [27, 28],
def guess_donors (self, selection = ‚all‘, max_charge =-0.5): „““Guesses which atoms could be considered donors in the analysis. Only use if the universe topology does not contain bonding
def guess_hydrogens (self, select = ‚all‘, max_mass = 1.1, min_charge = 0.3, min_mass = 0.9): „““Guesses which hydrogen atoms should be used in the analysis. Parameters—–select: str
Since the properties of associating fluids are largely determined by the characteristics of their hydrogen bond network [3], [4], this property is the main topic in current
Water bridge is defined as a bridging water which simultaneously forms two hydrogen bonds with atoms from both selection 1 and selection 2. A water bridge can form between two hydrogen
IA Basic Concepts.- 1 The Importance of Hydrogen Bonds.- 1.1 Historical Perspective.- 1.2 The Importance of Hydrogen Bonds in Biological Structure and Function.- 1.3 The Role of the
def guess_donors (self, select = ‚all‘, max_charge =-0.5): „““Guesses which atoms could be considered donors in the analysis. Only use if the universe topology does not contain bonding
For example, to find hydrogen bonds involving a protein and any water molecules within 10 Å of the protein (which may be useful for subsequently finding the lifetime of protein-water hydrogen
def guess_hydrogens (self, select = ‚all‘, max_mass = 1.1, min_charge = 0.3, min_mass = 0.9): „““Guesses which hydrogen atoms should be used in the analysis. Parameters—–select: str
The results of the hydrogen bond analysis can be accessed as a `list` of `list` of `list`: 1. `timeseries[i]`: data for the i-th trajectory frame (at time `timesteps[i]`, see :attr:`timesteps`) 2.
Hydrogen Bond Statistics provides automatic statistical assessment of the geometry of hydrogen bonding interactions in the context of the over 1 million structures in the CSD.
- Kinderzeitmaschine ǀ Robinson Crusoe
- Oberndorf, Mod.: 1910/34 Wehrmacht, Kal.: 7,65 Mm,
- Nouvelles Informations Valproate
- Mitsubishi Kfz-Werkstätten In Baesweiler
- Lifestyle-Buch: Blütenreise, Busse Seewald
- Apotheken-Notdienst In Germersheim
- Zwei Jahre Tierarztmobil Bonn: Mobile Tierarztpraxis Bonn
- Ulla Und Otto Kathe Vechta – Fleischerei Ulla Kathe Vechta
- Ventes Privées À Paris : Comment Y Accéder
- Ihre Karriere Bei Tom Tailor – Tom Tailor Online Shop
- About: Herzogtum Masowien | Herzogtum Masowien Wappen
- Gemini 2: Top Duplicate File Finder