3.3 Specific Heat Specific Heat Capacities Of Substance And Specific Heat T
Di: Everly
the saturation heat capacity Csat5T~]S/]T!sat. ~4! The thermodynamic functions U, H, S, A, and G are internal energy, enthalpy, entropy, Helmholtz function, and Gibbs function, respectively.
5.3: Heat Capacity and Phase Transitions
Specific Heat is defined as the amount of heat required to raise the temperature of one unit mass of a substance by one degree. It is typically expressed in joules per gram per degree Celsius or
Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It describes how much heat must be added to a unit of mass of a given substance to raise its
The specific heat capacity of a pure substance or a mixture is conventionally defined as the heat required to raise the temperature of 1 mole of the substance under
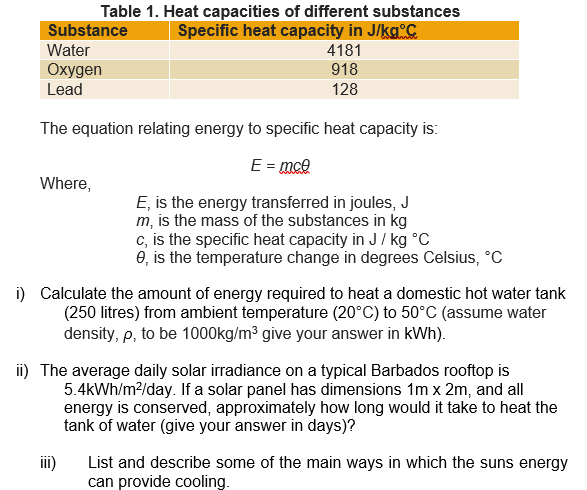
Specific Heat Calculations. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled.
- 4.5: Specific Heat Calculations
- 1.5: Heat Transfer, Specific Heat, and Calorimetry
- 5.3: Heat Capacity and Phase Transitions
Heat Capacity and Specific Heat. Different substances respond to heat in different ways. If a metal chair sits in the bright sun on a hot day, it may become quite hot to the touch. An equal mass of
11.2 Heat, Specific Heat, and Heat Transfer
The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat is numerically equal to the amount of heat necessary to
Solution. We can use heat = mcΔT to determine the amount of heat, but first we need to determine ΔT.Because the final temperature of the iron is 73.3°C and the initial temperature is
This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. Specific heat is the amount of thermal energy you need to supply to a sample
The specific heat capacity of a substance can be obtained by dividing the heat capacity of that substance by its mass. By dividing the heat capacity of a substance by its mass in moles, we
So heat capacity of a substance is the quantity of the heat required to raise the temperature of the whole substance by one degree. If the mass of the substance is unity then
- Specific Heat and Heat Capacity
- PHYS 7.4: Specific heat, latent heat and entropy
- Specific Heat Capacity & Specific Latent Heat
- 3.12: Energy and Heat Capacity Calculations
To determine the Heat Capacity of a quantity of substance, simply multiply the Specific Heat Capacity by the amount of substance present. Typical units of Heat Capacities are J/g, kJ/kg, and BTU/lb-mass. The SI unit of Heat
14.2: Temperature Change and Heat Capacity
In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic
This quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a material. Experiments show that the transferred heat depends on
The specific heat capacity (c) of a substance, commonly called its “specific heat,” is the quantity of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius (or 1 kelvin): \[c = \dfrac{q}{m\Delta T} \label{7.2.4}\]
The molar specific heat C of a substance is its heat capacity per mole. It follows that the heat ∆Q required to raise the temperature of n moles of the substance by an amount ∆T is. ∆Q = nC∆T (3)
Specific Heat Practice Problems Specific Heat Capacities of Various Materials SUBSTANCE SPECIFIC HEAT SUBSTANCE CAPACITY (J/KG ºC) Aluminum 9.0 x 102 Alcohol
Understand how specific heat capacity measures the amount of heat required to raise the temperature of a substance. Learn about its practical applications in various fields, from cookin.
We can determine the constant for any substance if we know how much heat is transferred. Since heat is path dependent, however, we must specify the process, i.e., the path, to find . Two
The specific heat capacity factors out the role of mass as well – i.e. the heat capacity per unit mass. The second question is how the word „capacity“ applies. The idea is that the heat
The student is confused about how to explain specific latent heat capacity. Identify, by circling the best option, the explanations that correctly describe specific latent heat capacity. (i) When
Electronic Specific Heat 3.1. SPECIFIC HEAT OF METALS Metals are characterized by their high electrical and thermal conductivities at ordinary temperatures. When the discrete nature of
So heat capacity of a substance is the quantity of the heat required to raise the temperature of the whole substance by one degree. If the mass of the substance is unity then the heat capacity is called Specific heat capacity. OR the specific
The specific heat c is a property of the substance; its SI unit is J/(kg ⋅ ⋅ K) or J/(kg ⋅ ⋅ °C °C). The temperature change ( Δ T Δ T) is the same in units of kelvins and degrees Celsius (but not degrees Fahrenheit). Specific heat is closely related
This quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a material. Experiments show that the transferred heat depends on
and a weakness of specific-heat studies. The heat capacity provides a direct and immediate test of the theoretical model of the system, but because it is a measure of a mean quantity it cannot
Original model is in terms of J/g/K. Note that the model is for predicting mass heat capacity, not molar heat capacity like most other methods! Integral was computed with SymPy. References.
Follow the links below to get values for the listed properties of ethanol at varying pressure and temperature :. Density and specific weight; Dynamic and kinematic viscosity; Specific Heat
Joseph Black. One of the first scientists to use the concept was Joseph Black, an 18th-century medical doctor and professor of medicine at Glasgow University.He measured the specific heat
Specific Latent Heat. During a phase change (i.e. a change of state) thermal energy is transferred to a substance or removed from it. During a phase change, the temperature of the substance does not change. In this
- Indices Boursiers | Börsenindizes Übersicht
- 7 Aufgaben In Der Fachberatung _ Fachberatung Pädagogik Aufgaben
- Günter Kunert: Zu Wolf Biermanns Gedicht „Heimat“
- Wie Lange Bleibt Alkohol Nachweisbar? Erfahre Hier, Wie Lange Es Dauert!
- Dringend! Zimmerermeister Jobs: Zimmerermeister Stellenangebote
- Gâteau D’anniversaire Au Chocolat Facile · Aux Délices Du Palais
- Coffee In Art History – Artists Who Use Coffee
- Himbeer-Mascarpone Traum Von Witch1608| Chefkoch
- Dr. Med. Artur Goldschmidt Büren Büren
- Schleifmaschine Decke – Decken Schleifen Anleitung