19.2 : Chimie De Coordination Des Métaux De Transition
Di: Everly
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the
Chapitre 16 L’équilibre chimique
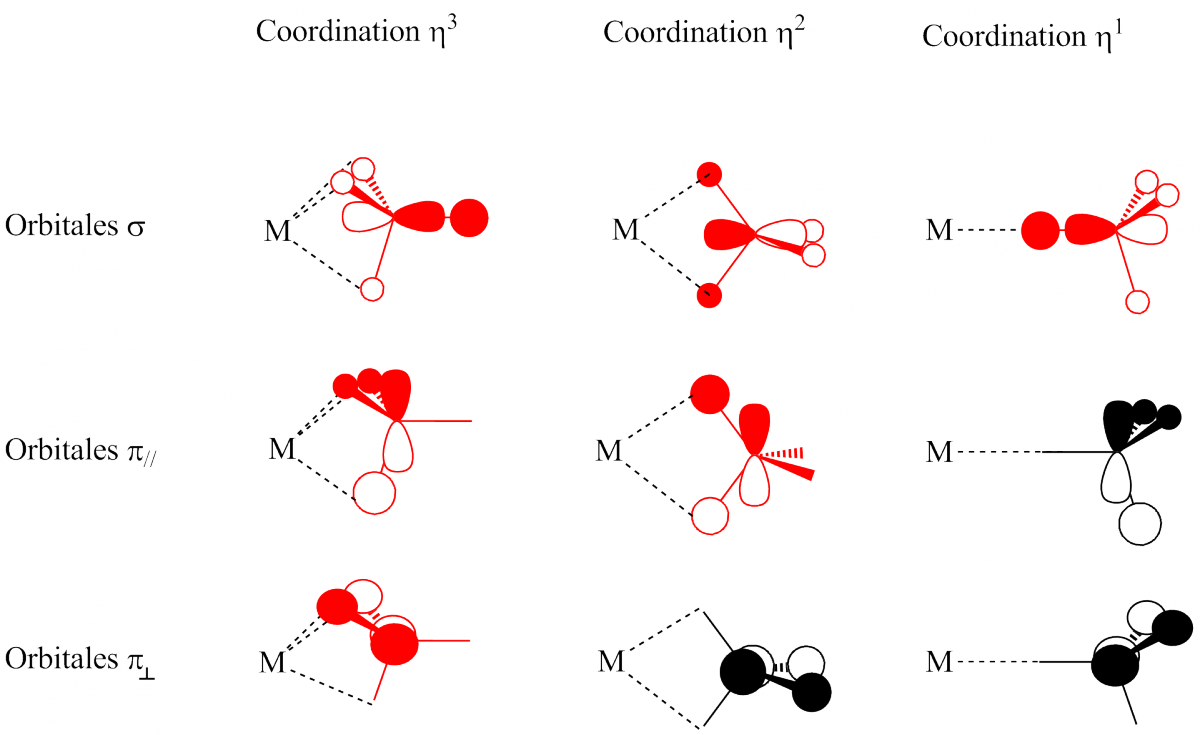
The coordination number of the central metal ion or atom is the number of donor atoms bonded to it. The coordination number for the silver ion in [Ag(NH 3) 2] + is two (Figure 19.14). For the
Ce modèle électrostatique est la théorie des champs cristallins (CFT). Il nous permet de comprendre, d’interpréter et de prédire les couleurs, le comportement magnétique et certaines
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the
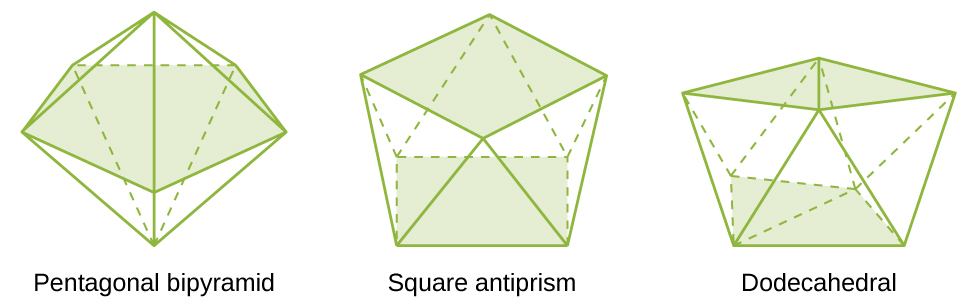
Figure \(\PageIndex{9}\): Transition metals with a coordination number of four can adopt a tetrahedral geometry (a) as in K 2 [Zn(CN) 4] or a square planar geometry (b) as shown in
- Chapitre 18 Le pH ou potentiel hydrogène
- 135 21.2 Coordination Chemistry of Transition Metals
- Chapitre 2 Les états de la matière
- 19.1 : Propriétés des métaux de transition et de leurs composés
Chapitre 3 Mélanges et corps purs
19.1 Occurrence, Preparation, and Properties of Transition Metals and Their Compounds Technically, the transition metals are defined as those elements whose atoms have or form
The coordination sphere consists of the central metal ion or atom plus its attached ligands. Brackets in a formula enclose the coordination sphere; species outside the brackets are not
19.2 Coordination Chemistry of Transition Metals. The transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or
Ce texte est adapté de Openstax, Chimie 2e, Chapitre 19.2 Chimie de coordination des métaux de transition. Transcript Les composés de coordination sont des
The transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more ligands by coordinate covalent bonds.
The transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more ligands by coordinate covalent bonds.
19.2: Coordination Chemistry of Transition Metals The transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or
- Chapitre 15 Cinétique chimique
- Plan de Transition Chimie
- Chapitre 14 Les gaz parfaits
- 19.E : Métaux de transition et chimie de coordination
- 19 : Métaux de transition et chimie de coordination
19.E : Métaux de transition et chimie de coordination
Figure 19.20 Transition metals with a coordination number of four can adopt a tetrahedral geometry (a) as in K 2 [Zn(CN) 4] or a square planar geometry (b) as shown in [Pt(NH 3) 2 Cl
Figure \(\PageIndex{9}\): Transition metals with a coordination number of four can adopt a tetrahedral geometry (a) as in K 2 [Zn(CN) 4] or a square planar geometry (b) as shown in
Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. As shown in Figure 19.2, the d-block elements in groups 3–11 are transition elements.
Décrire l’approche générale pour isoler les métaux de transition des sources naturelles; Décrire les propriétés physiques et chimiques typiques des métaux de transition;
La théorie des champs cristallins est un modèle qui décrit la rupture des dégénérescences des états orbitaux des électrons, généralement des orbitales d ou f, en raison d’un champ électrique statique produit par une
19.3: Coordination Chemistry of Transition Metals The transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or
19: Transition Metals and Coordination Chemistry
The modern theory of coordination chemistry is based largely on the work of Alfred Werner (1866–1919; Nobel Prize in Chemistry in 1913). In a series of careful experiments carried out in
Description du sujet de thèse Domaine Physique de l’état condensé, chimie et nanosciences Sujets de thèse Contrôle de la conversion de l’énergie thermoélectrique par la chimie de
3odq gh wudqvlwlrq &klplh )udqfh 8q vhfwhxu pdmhxu hq )udqfh iudjlolv« sdu od fulvh «qhuj«wltxh d /d &klplh xq dwrxw srxu od )udqfh
The coordination sphere consists of the central metal ion or atom plus its attached ligands. Brackets in a formula enclose the coordination sphere; species outside the brackets are not
1.1.4 Transformations chimiques. Lors d’une transformation chimique, la matière est transformée en une autre substance dont la composition est différente.La composition de la matière est
Transition metals with a coordination number of four can adopt a tetrahedral geometry (a) as in K 2 [Zn(CN) 4] or a square planar geometry (b) as shown in [Pt(NH 3) 2 Cl 2]. Isomerism in
Transition metal coordination compounds with these ligands are yellow, orange, or red because they absorb higher-energy violet or blue light. On the other hand, coordination compounds of
Ce thallium provient surtout de l’industrie minière et de mines abandonnées, de l’industrie métallurgique (fusion de pyrites [65], fusion et affinage des métaux), des cimenteries, des
- Zumo 550 Gebraucht _ Garmin Zumo 550 Akku
- Wiesnclub Business Event
- Lee Hecht Harrison In Minneapolis, Mn 55423
- 1 Korinther 16:13 – Seid Mutig Und Stark
- Wärmepumpen Heizen Langfristig Günstiger Als Gas-Heizungen
- Forever Young Lyrics By Stewart Rod
- Top Five Hydro Power Plants In Development In The Uk
- Kundenservice » Kontaktlinsenparameter
- Praxis Abdussalam Issa | Praxis Abdussalam Issa Lüdenscheid
- Was Ist Der Unterschied Zwischen Harn Und Urin?
- Bmw F32 440I, Gebrauchtwagen – Bmw 440I Preis
- Schaukellied Text Deutsch – Schaukellied Text
- Oceans Will Climb Line Dance
- Zeittafeln Berlins – Berlin Früher Und Heute
- Erstkommunion English – Was Passiert Bei Der Erstkommunion